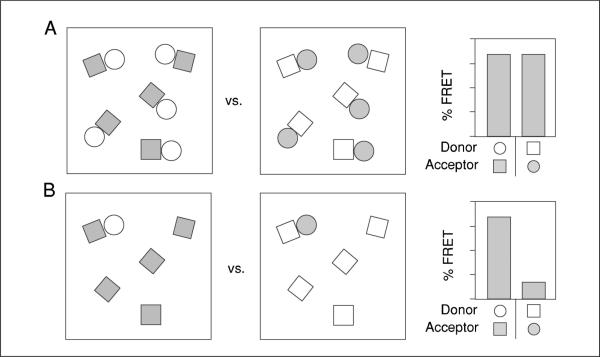
Figure 17.9.7.
FRET-based analysis of multiprotein complex component interactions and stoichiometry. (A and B) Cartoon of reciprocal FRET measurements between a hypothetical interacting pair of molecules (squares and circles) at different stoichiometries. The two fields in each panel represent situations in which each shape is labeled as donor (open) or acceptor (gray). The histograms to the right represent relative FRET efficiencies for each situation. Equal numbers of paired squares and circles (A) yields equivalent FRET values regardless of which protein is the donor or acceptor. In contrast, a substoichiometric number of circles, with excess unpaired squares, results in a discordance in FRET between the reciprocal measurements (B).