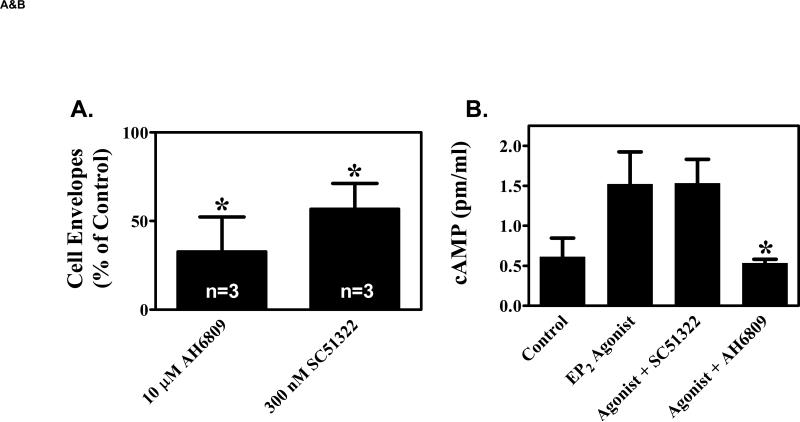
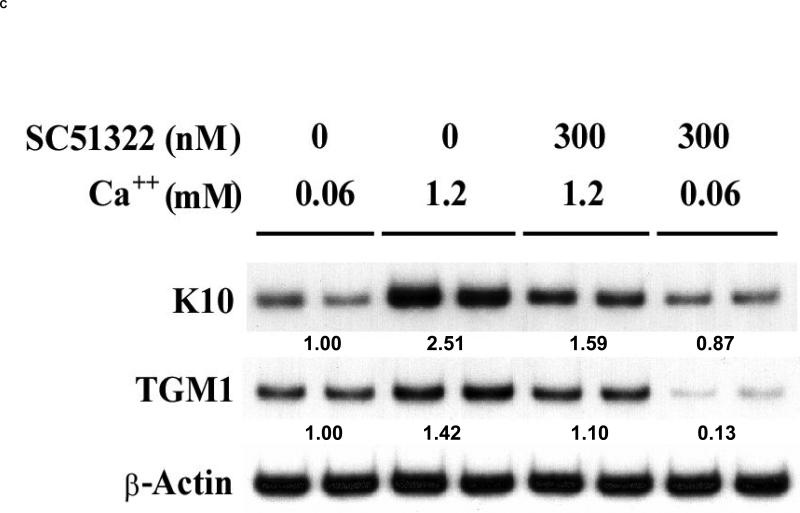
Figure 4. EP1 receptor antagonists inhibit keratinocyte differentiation.
(A). Primary human keratinocytes were grown in K-SFM (0.06 mM Ca2+). Addition of vehicle (0.1% DMSO), 10 μM of the non-specific EP1, EP2, EP3 receptor antagonist, AH6809, or 300 nM of the EP1 selective antagonist, SC51322 was begun when the cells were 50-60% confluent and every other day thereafter. At 3-4 days after reaching confluence, the capacity of the cells to form SDS-insoluble cornified cell envelopes was determined after trypsinizing the cells, pelleting the cells, and treating the cells for 3 hours with a calcium ionophore and high calcium media to stimulate envelope formation (envelope competence). The results were normalized to total cell counts and expressed as a percent of vehicle control cells. The results represent the mean and SEM of three experiments done in single or duplicate wells, with corneocytes from each well counted at least 10 times using a hemocytometer. * Results significantly different from control cells (P < 0.05; one sample t-test). (B). The non-specific EP1, EP2, EP3 antagonist (AH6809), but not the EP1 specific antagonist (SC51322), inhibits EP2 receptor stimulated cAMP production. Primary human keratinocytes were first incubated with 3 μg/ml indomethacin to block endogenous PGE2 formation. The cells were then stimulated with a highly selective EP2 receptor agonist (CAY10933, 10 nM) in the absence or presence of AH6809 (12.5 μM) and SC51322 (500 nM) for 15 minutes. Cyclic AMP was measured using a commercial EIA kit. (C). The EP1 specific agonist SC51322 inhibits calcium-dependent up-regulation of the differentiation specific markers, cytokeratin K10 and epidermal transglutaminase (TGMI). Duplicate wells of primary human keratinocytes were grown in K-SFM (0.06 mM Ca2+) until they reached near confluence (1 day prior to attaining confluence). At this time, the cells were treated with vehicle or 300 nM SC51322. One hour later, additional Ca2+ (1.2 mM), was added to the wells as indicated. After an additional 48 hours (1 day post-confluent), the cells were processed for isolation of total RNA. Semi-quantitative RT-PCR was then performed on each of the duplicate samples using specific primers for K10, TGM1, or β-actin (loading control). All PCR reactions were stopped during the exponential phase of PCR amplification and visualized by agarose gel electrophoresis and autoradiography. The mean normalized band intensity for both K10 and TGM1 (normalized to β-Actin) is shown under each duplicate radiographic image. In each case, the band intensity is shown as a ratio compared with the low calcium (0.06 mM) vehicle control cells (assigned a value of 1.00). Band intensity was determined by area integration using NIH Image J software.