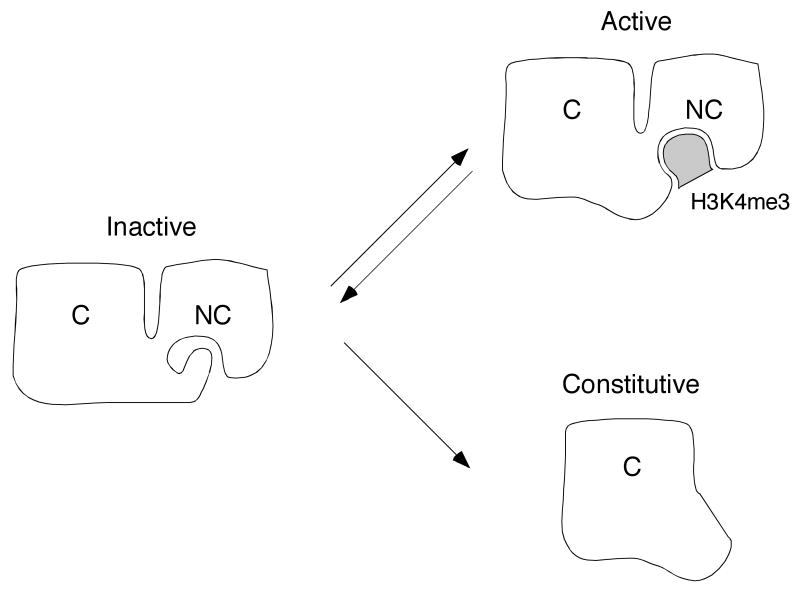
Fig. 2.
A model for allosteric activation of the RAG complex by modified histone H3. White figures represent RAG-2; C and NC denote core and non-core regions. respectively. Shaded object represents trimethylated lysine 4 of histone N3 (H3K4me3). In a hypothetical inactive conformation (left), the aromatic channel of the RAG-2 PHD finger is occupied by an inhibitory domain residing elsewhere in the non-core region. In the hypothetical active conformation (upper right), the PHD finger is bound by histone H3K4me3 and the putative inhibitory domain is released. The RAG-2 core fragment (lower right) lacks both the PHD finger and the putative inhibitory domain. In this configuration RAG-2 is proposed to assume an active configuration constitutively. For further discussion, see text.