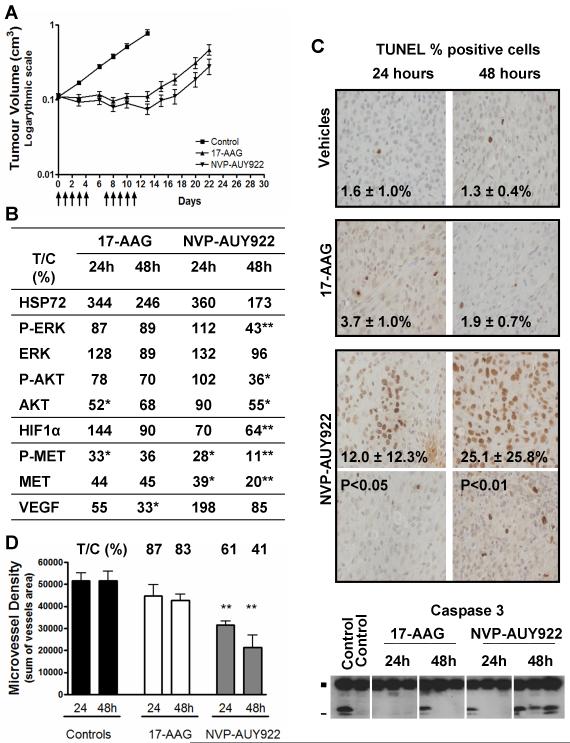
Figure 6. In vivo effect of NVP-AUY922 and 17-AAG in the adult U87MG GB subcutaneous tumor xenografts.
Control mice received vehicle only (10% DMSO, 5% Tween 20, 85% saline as control for NVP-AUY922, and 43% ethanol, 33% propylene glycol, 24% cremaphor as control for 17-AAG). Treated mice received either 80 mg/kg ip once daily 17-AAG (maximal tolerated dose) or 50 mg/kg once daily NVP-AUY922 (below the maximal tolerated dose). A- Antitumor activity of a 2 week treatment (5d/7) by either NVP-AUY922 (▼) or 17-AAG (▲), compared to vehicle controls (■). Arrows (↑) indicate the days of drug administration. B- Biomarker modulation using protein quantification by electrochemiluminescent immunoassay (MesoScale Discovery) except for VEGF which was determined by ELISA. Results are given as the percentage of protein level in the treated group as compared to control group at the corresponding time point (% T/C). C- Apoptosis was assessed by caspase 3 cleavage (immunoblotting; ■ full length inactive and - cleaved active fragments) and DNA fragmentation analysis (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, TUNEL). Representative images are shown at 40x objective magnification for each treatment group, at 24 or 48hrs after a 3 day-treatment course. Two panels are shown for the NVP-AUY922 group as distribution of TUNEL positive cell throughout each sample were unequal. Values shown are mean of percentage of positive cells in 8 fields of view. D- Microvessel density analysis by immunohistochemistry staining in mouse endothelial cells by rat anti-CD34 antibody. The histograms shows results expressed as the sum of vessel areas in the fields of view (x5 objective magnitude). Significant P <0.05 (*) and <0.01 (**). Above the histograms, the values show percent treated over control (% T/C) for the drug treatment.