Abstract
Mycobacterial suppression of central nervous system (CNS) autoimmunity has been demonstrated in various experimental models, epidemiological studies, and clinical trials. Recent studies have led to an increased understanding of the cellular and molecular interactions involved in the pathogenesis of autoimmune diseases and of mycobacterial immunity. Here, we review some of the mechanisms by which mycobacterial infection might modulate the clinical course of CNS autoimmunity. A more complete understanding of these mechanisms may lead to the development of novel immunotherapeutic tools for treating autoimmune diseases.
Keywords: central nervous system, autoimmunity, mycobacterium
Immunity to mycobacterial infection
Mycobacteria comprises about 80 species, including Mycobacterium tuberculosis (Mtb), a human pathogen that causes approximately two to three million deaths per annum. Statistics suggest that one third of the world’s population is currently infected with Mtb, and around 100 million people are annually vaccinated with a live attenuated mycobacterium called bacillus Calmette-Guerin (BCG). Additionally, owing to the prevalence of saprophytic mycobacteria in the environment, the human immune system is constantly exposed to mycobacterial infection (Rook et al. 2004). Mycobacteria are intracellular pathogens, and their survival fundamentally depends on circumventing host protective immune responses. Therefore, mycobacteria have developed strategies to dampen host immunity. Moreover, growing numbers of epidemiological and clinical studies suggest that infection with mycobacteria or vaccination with BCG, especially in early childhood, may beneficially contribute to the development of normal immune regulation, which plays an important role in balancing pro- and anti-inflammatory signals to resolve inflammatory events (Rook et al. 2004; Yazdanbakhsh et al. 2002). Understanding the mechanisms by which mycobacteria evade host immune responses and modulate inflammatory responses may assist in the development of potent vaccines against Mtb and may be applied to the treatment of autoimmune diseases.
Mycobacteria-induced subversion of innate immune responses has been demonstrated in multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). Here, we review the immunomodulary role of mycobacteria in both MS and EAE. In addition, we summarize some of the possible mechanisms by which mycobacterial infections induce immunity that provides protection from autoimmune diseases. Ultimately, knowledge of these mechanisms will aid in the development of new immune therapies for the treatment of autoimmune diseases.
Innate immunity
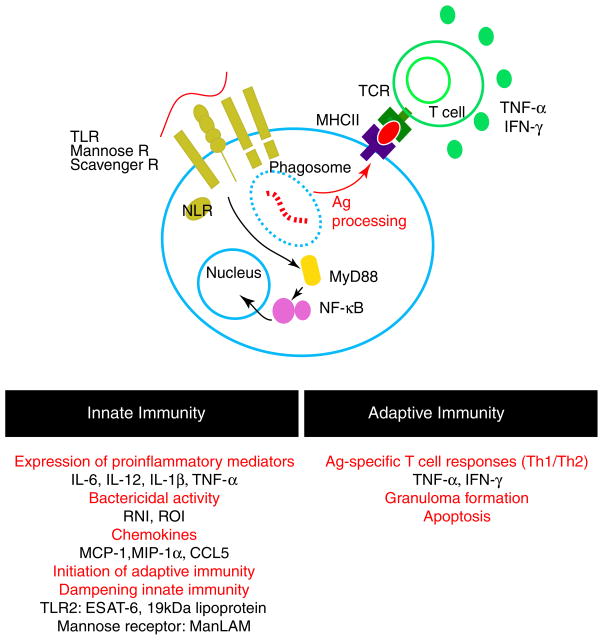
The initial recognition of mycobacteria by the host immune system is mediated by pattern-recognition receptors such as toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, and C-type lectins, including the mannose receptor (CD207), the dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN/CD209), and Dectin-1 expressed by phagocytes (Jo 2008). The initial interaction triggers intracellular signaling cascades (Fig. 1), which coordinate innate and adaptive immune mechanisms and influence both the magnitude and the quality of responses against mycobacterial infection. In mice, mycobacterial infection triggers a TLR2-mediated signaling pathway, which directs a nitric oxide (NO)-dependent effector cascade (Thoma-Uszynski et al 2001) and cell activation by mycobacterial cell wall lipoglycans such as phosphoinositol-capped lipoarabinomannan, phosphatidyl myo-inositol mannosides (PIM2 and PIM6), or the 19-kDa mycobacterial lipoprotein (Gilleron et al. 2003; Means et al. 1999; Quesniaux et al. 2004). In general, engagement of TLRs activates the adaptor molecule tumor necrosis factor (TNF) receptor-associated factor-6, which culminates in the activation of mitogen-activated protein kinases and the transcription factor NF-κB (Cook et al. 2004), leading to the production of inflammatory mediators such as nitric oxide and proinflammatory cytokines such as TNF, interleukin (IL)-1, and IL-12. Studies have shown that long-term control of M. tuberculosis infection is both TLR2- and TLR4-dependent in mice (Abel et al. 2002; Drennan et al. 2004) and that innate immune responses elicited by mycobacterial components are critical for protective host immune responses.
Fig. 1.
Immune responses against mycobacterial infection. Mycobacteria, recognized by scavenger, mannose, and toll-like receptors, are phagocytosed to eliminate bacilli and processed for T-cell presentation, thus mounting adaptive immune responses. Immediate responses by the innate immune system have a decisive influence on host adaptive response against mycobacterial infection. The characteristic adaptive immune response against mycobacterial infection includes strong Th1 responses and granuloma formation
Paradoxically, mycobacterial components may also dampen innate immune responses. It has been shown that binding of mycobacterium-produced Early Secreted Antigenic Target protein 6 (ESAT6) to TLR2 interferes with MyD88-dependent signaling cascades and inhibits activation of NF-κB (Pathak et al. 2007). Therefore, the engagement of TLR2 by ESAT6 attenuates innate immune responses and the production of IL-12, TNF, and NO, in addition to suppressing various other critical proinflammatory mediators (Pathak et al 2007). The 19 kDa mycobacterial lipoprotein has been shown to inhibit major histocompatibility complex class II expression on macrophages and reduce interferon (IFN)-γ responsiveness of macrophage in a TLR2-dependent manner (Pai et al. 2003). It appears that the proinflammatory activity via TLR2 is attributed to mycobacterial ligands containing tri-and tetra-acylated forms of lipomannans. Both the mannose receptor and DC-SIGN interact with di-acylated lipomannans exhibiting a potent inhibitory effect on cytokine and nitric oxide secretion (Geijtenbeek et al. 2003; Nigou et al. 2001). This attenuation of innate immunity and subsequent adaptive immune responses by mycobacterial components is likely associated with the persistent survival of mycobacteria in the host. Subverting the innate immune responses may be an underlying mechanism of mycobacteria-induced protection against MS and EAE.
A number of studies have provided evidence of marked expression of TLRs during central nervous system (CNS) autoimmune responses in both human multiple sclerosis lesions and in rodent EAE lesions. Zekki et al. (2002) showed that robust expression of TLR2 and CD14 transcripts occurred in the CNS following myelin oligodendrocyte glycoprotein (MOG) immunization in EAE mice and identified microglia/macrophages as the main cellular source of TLR2 in the brain of these EAE animals. Similarly, Visser et al. (2006) found that a distinct phagocyte subset, including granulocytes, macrophages, and dendritic cells, contained peptidoglycan (PGN), an alternative TLR ligand in both human multiple sclerosis and primate EAE brain tissue. The number of PGN-containing phagocytes was significantly higher in EAE animals compared to controls and was highest in acute phase EAE. The presence of PGN in the inflamed brain suggests that the binding of this ligand to its recognition receptors, namely TLR2, Nod1, Nod2, and TLR2/6, in association with CD14, may play an important role in promoting CNS autoimmunity. Another study, by Farez et al. (2009), found increased serum levels of 15-α-hydroxicholestene in both progressive MS and murine EAE subjects. These levels promoted neuroinflammation by activating microglia, macrophages, and astrocytes via a TLR2/poly (ADP-ribose) polymerase-1 (PARP1) dependent pathway. This suggested that the TLR2/PARP1 pathway itself was a potential new target to treat progressive autoimmune disease of the CNS.
As the above studies suggest, many pattern-recognition receptors have a significant potential to modulate autoimmune mechanisms in the CNS. We also know that a variety of mycobacterial components can act through these same receptors during the initial interactions between host and bacteria. Thus, these mycobacterial agents have the potential to shape immune responses towards both proinflammatory and tolerizing directions. Since the primary target cells of mycobacterium overlap with the phagocytes that govern autoimmune inflammation in the CNS, mycobacterial components potentially compete with host-derived, neuroinflammation-boosting agents for the same receptors during concomitant immune responses. Although it is not completely understood how mycobacteria express anti-EAE activity or even whether or not this happens at the level of the innate immune mechanism, there is certainly potential for interactions via pattern-recognition receptors expressed by phagocytes in the CNS. Further studies need to explore these potential interactions to elucidate the contribution of mycobacteria to long-term protective immune responses against the development and progression of EAE autoimmunity and to validate their applicability as general immune modulators.
Adaptive immunity
Protective immunity against mycobacteria depends on Th1 responses, largely coordinated by IFN-γ and TNF-α. CD4+ and CD8+ T cells produce IFN-γ, which controls bacterial growth by inducing macrophage activation and thus production of TNF-α and bactericidal effector molecules, including reactive nitrogen intermediates (RNI) (Cooper et al. 1993; Xing et al. 2001) (Fig. 1). IFN-γ is also essential in governance of protective granuloma formation. TNF-α, in synergy with IFN-γ, induces RNI production which directs effector cells to the site of infection by influencing the expression of adhesion molecules and chemokines; this helps maintain the integrity of the granuloma structure. Granuloma formation is a hallmark of an effective, protective immune response against mycobacterial infection (Co et al. 2004; Raupach and Kaufmann 2001). Briefly, upon infection, the immune system recruits a collection of mononuclear phagocytes, which surround individually infected macrophages, T cells, B cells, DCs, neutrophils, and fibroblasts and promote production of extracellular matrix components (Cosma et al. 2003; Peters and Ernst 2003). The mycobacterial granuloma is a tightly integrated structure due to the differentiation of macrophages into giant multinuclear epithelioid cells. Thus, granulomas physically prevent dissemination of bacteria as well as control inflammation and tissue pathology. Additionally, granulomas directly reduce the number of bacteria by multiple mechanisms including production of reactive oxygen intermediates and RNI. Although a granuloma is a physically integrated entity, its component units are dynamically replenished by a continuous bidirectional flux of cells (Co et al. 2006). The trafficking of immune cells into granulomas is guided by a wide range of chemokines, such as CCL2, CCL3, CCL5, CXCL9, and CXCL10 (Algood et al. 2003), and this leukocyte trafficking modifies concomitant immune responses (Co et al. 2006; Sewell et al. 2003).
Apoptosis is another important mechanism of protective immunity against mycobacterial infection. Experiments with IFN-γ-deficient mice (which are also deficient for nitric oxide production) or mice treated with NO inhibitors demonstrate that the absence of IFN-γ or NO and decreased apoptosis in these models lead to earlier death. However, protection in these animals fails due to uncontrolled lymphocyte activation, not because of uncontrolled bacterial growth. This demonstrates that contraction of the lymphocyte compartment is a crucial stage of the protective immune response against mycobacterial infections (Cooper et al. 2002; Cooper et al. 1993). The dual role of IFN-γ and NO in promoting and limiting T-cell activation also contributes to regulating undesirable self-reactive T-cell responses.
Mycobacterium in the CNS autoimmune disease model EAE
The suppressive effect of mycobacteria in the development of autoimmune responses has been implicated in various experimental autoimmune disease models, including experimental autoimmune encephalomyelitis. A study in the 1960s demonstrated that Lewis rats are resistant to the induction of EAE when pretreated with complete Freund’s adjuvant (CFA). The protective effect of mycobacteria on EAE has been demonstrated with immunization using heat-killed Mtb, live and heat-killed BCG, or purified protein derivative (PPD) from Mtb. The suppressive effect of heat-killed Mtb on EAE is most pronounced when compared with the suppressive effect of other bacteria such as Escherichia coli, Shigella, and Staphylococcus aureus and can be achieved using PPD from many different strains of mycobacteria, such as Mycobacterium avium, Mycobacterium kansaii, Mycobacterium intracellulare, and Mycobacterium bovis (Ben-Nun et al. 1993; Lehmann and Ben-Nun 1992). Subcutaneous (s.c.) or intraperitoneal (i.p.) immunization of mice with CFA or heat-killed Mtb 4 weeks prior to EAE induction confers inhibition of EAE clinical symptoms. This inhibition is dose-dependent with heat-killed Mtb and has been shown to be effective when the immunization is given i.p. or s.c. before or up to 2 days post EAE induction. Recently, it has also been demonstrated that the mycobacteria-induced protection against EAE can be conferred by prior intracerebral (i.c.) infection with live BCG (Lee et al. 2008). Infection of the CNS with BCG 21 days prior to EAE induction with MOG35–55 in CFA prevents the development of EAE clinical symptoms and pathology. This study clearly indicates that ongoing anti-BCG Th1 and Th17 responses do not accelerate the induction of T-cell responses specific for CNS self-Ag in spite of being in the same anatomical compartment; rather, these ongoing anti-BCG Th1 and Th17 responses appear to inhibit autoimmune responses. All of these results using experimental models report that mycobacteria are beneficial against EAE development and progression. Possible mechanisms for this protection are described in the following section.
Proposed regulatory mechanisms induced by mycobacteria in autoimmune disease models
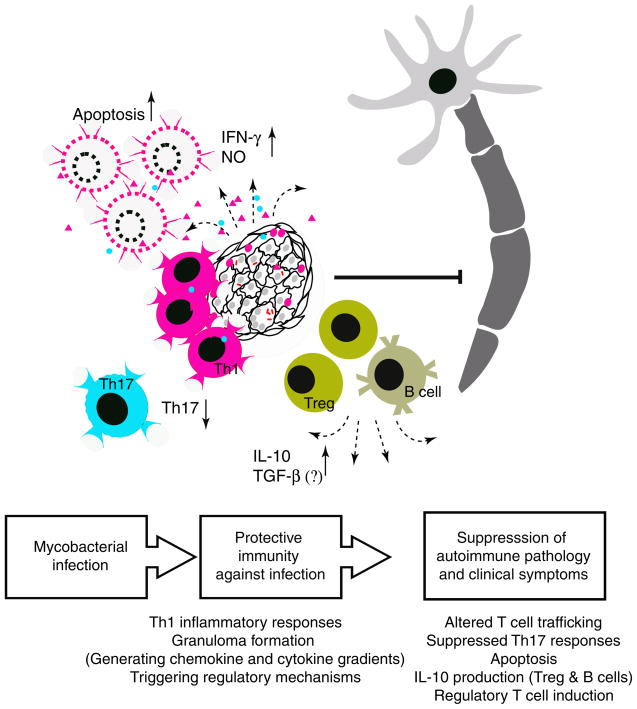
The reduction of self-Ag-specific responses at the effector site appears to be the major mechanism by which mycobacterial infection suppresses autoimmune responses. This feature is mostly attributed to characteristic anti-mycobacterial immune responses, such as strong IFN-γ responses, apoptosis, and granuloma formation.
Altered T-cell trafficking from the CNS to the peripheral inflammatory sites
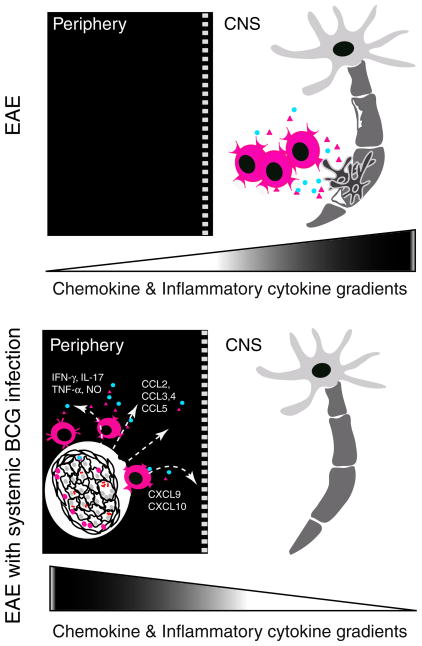
The concept that the BCG-induced granuloma acts as a sink for lymphocytes was introduced by our group (Sandor et al. 2003; Sewell et al. 2002) (Fig. 2) and was based on findings that a variety of T cell receptor (TCR) specificities could be found in granulomas and that BCG-nonspecific T cells also contributed to this diversity (Hogan et al. 2001; Sewell et al. 2003; Co et al. 2006). The influx of BCG-nonspecific T cells not only directly influences the local protective immune responses against infection, but it also modifies the progression and results of concomitant immune responses (Sewell et al. 2003). A former study from our group demonstrated that CD4+ T-cell-mediated autoimmune responses in the CNS could be regulated by i.p. BCG infection using the EAE model. BCG-infected animals had fewer self-Ag (MOG35–55)-specific IFN-γ-producing CD4+ T cells in the brain compared to control EAE animals (Sewell et al. 2003). Surprisingly, MOG-specific CD4+ T cells were found to infiltrate into mycobacterial granulomas formed at a distant site, in the liver. The diverted trafficking of MOG-specific T cells from their original effector site to the periphery led to reduced EAE clinical symptoms and tissue pathology in i.p. BCG-infected animals (Sewell et al. 2003).
Fig. 2.
Systemic BCG infection leads to altered T-cell trafficking, thus suppressing a CNS autoimmune disease. Prior infection also reduces EAE clinical symptoms and the frequency of IFN-γ-producing MOG-specific T cells in the CNS. As a result of the chemokine and cytokine gradients generated by peripheral granuloma, activated auto-reactive T cells are diverted from their original effector sites and are detained in granuloma
The diverted trafficking of MOG-specific T cells suggests that infection-induced inflammatory responses have the potential to redirect and detain pathogenic self-Ag-specific T cells, thereby preventing them from participating in an inflammatory response that is spatially distinct. This appears to be one of the key mechanisms by which infection regulates autoimmune responses. As a matter of fact, in another infection model, infection with lymphocytic choriomeningitis virus prevents diabetes in prediabetic mice by redistributing pathogenic autoimmune T cells from the islets to the pancreatic draining lymph node (Christen et al. 2004). Altogether, these data demonstrate that one inflammatory response can completely reshape another concomitant immune response.
Suppression of the Th17 response
IL-17 is important in chemotaxis of neutrophils through granulopoiesis and α-chemokines (CXC) chemokine induction (Laan et al. 1999; Kolls and Lindén 2004; Yu et al. 2007), proinflammatory cytokine secretion, and production of metalloproteases, which mediate tissue destruction (Agarwal et al. 2008; Miossec 2003). Th17 cells are therefore crucial in regulating tissue inflammation and pathology. Many studies have indicated that Th17 cells play a crucial role in autoimmune tissue pathology. IL-17 was shown to be directly involved in the destruction of cartilage and bone in rheumatoid arthritis patients (Bush et al. 2002). Moreover, IL-17 deficiency or treatment with IL-17 receptor antagonists prevented development of adjuvant-induced arthritis in mice. Additionally, IL-17-deficient mice (Komiyama et al. 2006) or mice treated with an IL-17 blocking antibody (Hofstetter et al. 2005) developed resistance to the development of EAE. These clinical and experimental data suggest a pathological role of IL-17 in autoimmune diseases (Bettelli et al. 2007).
Recently, we demonstrated that after BCG infection, which conferred protection against EAE, IL-17 responses were suppressed in EAE mice. Protected mice had significantly suppressed IL-17 CD4+ T-cell responses in the brain compared to control EAE mice. In contrast, IFN-γ responses in the brain were equivalent in i.c. BCG-infected and control EAE mice (Lee et al 2008). These data, together with studies showing that IFN-γ cannot always downregulate IL17 production and may in fact contribute to the pathogeneic function of Th17 cells (Chen et al. 2006; McGeachy et al. 2007; Annunziato et al. 2007), suggest that suppression of IL-17 responses, and not IFN-γ responses, confers protection against EAE. Clearly, BCG given prior to EAE induction suppresses IL-17, one of the most crucial cytokines promoting tissue pathology, thus inhibiting EAE. Interestingly, the suppressive effect observed in i.c. BCG-infected mice is more pronounced than that obtained in i.p. BCG-infected mice (Sewell et al. 2003; Lee et al. 2008), suggesting that BCG-induced immune responses in the brain suppress autoimmune responses in ways distinct from diversion of encephalitogenic T cells to granulomas, which are formed in the brain upon i.c. BCG infection. Additionally, the observed protection demonstrates that BCG-induced regulation of autoimmune responses might be attributed to a qualitative change in T-cell responses and supports the notion that BCG may act as an active regulator of IL-17 secretion in EAE.
Although the current studies on IL-17 responses have been largely focused on CD4+ αβ T cells, other cell types such as γδ T cells, NK cells, and neutrophils also produce IL-17. Especially, γδ T cells appear to be a major source of IL-17 after E. coli (Shibata et al. 2007) or pulmonary BCG infection (Umemura et al. 2007; Roark et al. 2008). Therefore, it is of great interest to understand how BCG infection modulates IL-17 responses by other cell types, especially γδ T cells, and suppresses autoimmune responses in the CNS during the course of EAE.
Immunomodulatory role of mycobacterium by inducing apoptosis of activated cells
Apoptosis is a critical regulatory mechanism that helps to eliminate activated pathogenic effector cells, thereby constraining inflammatory responses and preventing excessive inflammation. It is well established that IFN-γ crucially controls apoptosis of activated CD4+ T cells during mycobacteria infection (Cooper et al. 2002; Dalton et al. 2000; Gilbertson et al. 1999). IFN-γ coordinates various apoptotic pathways to reduce activated CD4+ T cells by regulating mitochondrial damage-mediated apoptosis, such as Bcl-2, Bax, Bim, Bid, Apaf-1, and caspase-9, or by promoting cell-extrinsic signals of apoptosis such as TRAIL, DR5, and TNFR1 (Li et al. 2007b). The ability of IFN-γ to coordinate these apoptotic pathways demonstrates the potential of IFN-γ to mediate immune regulatory mechanisms in autoimmune disease (Chu et al. 2000; O’Connor et al. 2005; Qin et al. 2004).
O’Connor and colleagues have demonstrated significantly more apoptosis of encephalitogenic MOG-specific CD4+ T cells transferred to BCG-infected mice compared to uninfected mice and that this bystander deletion of encephalitogenic CD4+ T cells resulted in reduced EAE clinical scores. They also found that IFN-γ receptors are required on donor CD4+ T cells for apoptosis to occur, clearly showing the critical role of IFN-γ in the apoptosis of encephalitogenic CD4+ T cells during BCG infection (O’Connor et al. 2005). Therefore, IFN-γ-induced apoptosis during mycobacterial infection accounts for at least some mycobacteria-mediated suppression of autoimmune responses.
Apoptosis-mediated regulation of autoimmune responses has been shown in another autoimmune disease model (Qin et al. 2004). Splenocytes from BCG-infected NOD mice were less diabetogenic when transferred to NOD/SCID mice compared with non-infected NOD donors. This effect was reversed when anti-IFN-γ Ab was administered during BCG infection. Increased apoptosis was accompanied by the upregulation of Fas, Fas ligand, and TNFR on T cells and was reversed when anti-IFN-γ Ab was administered during BCG infection, suggesting that IFN-γ was mediating deletion of diabetogenic T cells and protecting against disease (Qin et al. 2004). The studies of experimental autoimmune disease with concomitant BCG infection described above clearly demonstrate the beneficial role of BCG-induced inflammatory responses to host immunity and recapitulate the disease-limiting role of IFN-γ during immune responses.
Regulatory T cells in mycobacterial infections
Studies have shown the induction of regulatory T cells during mycobacterial infection and the role of these regulatory T cells as anti-inflammatory regulators during mycobacterial infection (Li et al. 2007a; Ribeiro-Rodrigues et al. 2006; Scott-Browne et al. 2007). Regulatory T cells induced by chronic mycobacterial infection hinder efficient bacterial clearance (Kursar et al. 2007). However, mycobacteria are beneficial to the host because they prevent excessive inflammatory responses that would otherwise lead to detrimental tissue pathology (Sakaguchi et al. 2008) and because persisting mycobacteria contribute to memory T-cell generation, resulting in a fortified long-term protection. Regulatory T cells are critical to optimize host defense mechanisms, and mycobacterium-induced regulatory T cells may also interfere with or control other concurrent inflammatory responses. For example, using the ovalbumin (OVA)-induced eosinophilic airway inflammation model, Zuany-Amorim and colleagues demonstrated that the Mycobacterium vaccae –induced CD4+CD45RBLow regulatory T-cell population confers protection against airway inflammation in an IL-10- and TGF-β-dependent manner (Zuany-Amorim et al. 2002) when animals were immunized three weeks prior to OVA challenge.
The regulatory T-cell population involved in mycobacteria-induced protection has been studied in the insulin-dependent diabetes mellitus (IDDM) model (Qin et al. 1993, 2006). Qin and colleagues have shown that splenocytes from CFA-immunized mice can delay the onset of diabetes from nondiabetic NOD mice. However, this suppressive effect is abolished by depletion of CD4+ T cells, indicating the presence of a regulatory population in the CD4+ T-cell compartment (Qin et al. 1993). Further phenotypic characterization of the regulatory population from CFA-protected NOD mice has identified a CD4+CD8+ double-positive CD25+ T-cell subset (MT-5B). This T-cell subset suppresses in vitro T-cell proliferation in an Ag-nonspecific and contact-independent manner, which is mediated by granzyme B and perforin, similar to killer mechanisms governed by NK cells and cytotoxic T lymphocytes. Treatment of diabetogenic splenocytes with MT-5B supernatant prior to adoptive transfer impairs their ability to transfer diabetes (Qin et al. 2006). This suppression is mostly due to increased T-cell apoptosis in a granzyme B-dependent manner. These data support the idea that mycobacteria-induced regulatory T cells have the potential to suppress auto-reactive T cells.
Regulatory role of B cells
The regulatory role of B cells has been recognized in various autoimmune disease models (Fillatreau et al. 2002; Mauri and Ehrenstein 2008; Mauri et al. 2003; Mizoguchi et al. 2002). B-cell-deficient EAE mice exhibit a delayed induction of IL-10 in the CNS that inhibits recovery from EAE. This regulatory role appears to be mediated directly by IL-10-producing B lymphocytes. B cells may also regulate EAE autoimmunity through regulation of CD4+CD25+ T cells via B7 (Mann et al. 2007).
A recent study has demonstrated the contribution of a mycobacterial component to the induction of regulatory B lymphocytes. Mtb (contained in adjuvant used to induce autoimmune responses) triggers toll-like receptor signaling in B lymphocytes which stimulates IL-10-mediated regulatory function, thereby suppressing both Th1 and Th17 inflammatory responses (Lampropoulou et al. 2008). Although the IL-10-producing B-lymphocyte population has not been characterized in EAE models with mycobacteria pretreatment or treatment regimen, an early study using M. avium infection of NOD mice has shown that elevation in B lymphocytes rather than T lymphocytes is associated with long-term mycobacteria-induced protection (Martins and Aguas 1996). Further studies will elucidate the contribution of regulatory B lymphocytes in mycobacteria-mediated suppression of autoimmune responses.
Perspective: translation of the experimental models to human autoimmune diseases
Understanding the underlying mechanisms by which mycobacteria modulate autoimmune responses in the CNS in experimental models allows us to characterize essential components of those immune responses and therefore provides insight into the development of therapies targeting immune modulation in human diseases (Fig. 3). To date, the potential of mycobacteria immunization in controlling autoimmune responses has been evaluated (Classen and Classen 1999; Paolillo et al. 2003; Parent et al. 1997; Ristori et al. 1999; Sanjeevi et al. 2002). Human clinical trials using BCG vaccination as a treatment for autoimmune diseases and the long history of BCG vaccine in human medicine show that BCG is an attractive candidate for an effective and safe immune modulator. Ristori and colleagues have reported the effect of BCG vaccine as an immune modulator in MS. They performed a magnetic resonance imaging (MRI)-monitored trial with 14 relapsing–remitting MS patients and showed significantly reduced MRI activity in ten out of 12 patients and no adverse effects after a single crossover trial with BCG (Ristori et al. 1999).
Fig. 3.
Mycobacterial infection modifies autoimmune T-cell responses in the CNS. BCG infection modifies subsequent effector T-cell responses in the CNS by suppressing Th17 responses, inducing apoptosis in activated cells and generating regulatory T and B cells
The promising results from BCG vaccination clinical studies validate research on mycobacteria-induced suppression in human autoimmune diseases and strengthen the rationale for future controlled trials. Mechanistic studies regarding mycobacteria-induced suppression of autoimmune responses in the CNS provide us with new a perspective for understanding how one immune response facilitates, synergizes, or detracts from other concurrent or subsequent responses. Future studies will provide insight into novel immunological components involved in regulatory mechanisms that may potentially be targeted for therapeutic intervention.
Footnotes
Guarantor of the work: Zsuzsanna Fabry
Conflict of interest The authors have no financial or personal conflict of interest.
Contributor Information
JangEun Lee, Department of Pathology and Laboratory Medicine, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA. Cellular and Molecular Pathology Program, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA.
Matyas Sandor, Department of Pathology and Laboratory Medicine, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA. Cellular and Molecular Pathology Program, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA.
Erika Heninger, Department of Pathology and Laboratory Medicine, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA.
Zsuzsanna Fabry, Email: zfabry@wisc.edu, Department of Pathology and Laboratory Medicine, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA. Cellular and Molecular Pathology Program, School of Medicine and Public Health, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, USA.
References
- Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. Toll-like receptor 4 expression is required to control chronic mycobacterium tuberculosis infection in mice. J Immunol. 2002;169(6):3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol. 2008;35(3):515–519. [PubMed] [Google Scholar]
- Algood HM, Chan J, Flynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 2003;14(6):467–477. doi: 10.1016/s1359-6101(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204 (8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A, Yossefi S, Lehmann D. Protection against autoimmune disease by bacterial agents. Ii. Ppd and pertussis toxin as proteins active in protecting mice against experimental autoimmune encephalomyelitis. Eur J Immunol. 1993;23(3):689–696. doi: 10.1002/eji.1830230318. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(h)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor igg1 fc fusion protein. Arthritis Rheum. 2002;46(3):802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an ip-10 gradient. J Clin Invest. 2004;113(1):74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated cd4 t cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(1):123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen JB, Classen DC. Immunization in the first month of life may explain decline in incidence of iddm in the netherlands. Autoimmunity. 1999;31(1):43–45. doi: 10.3109/08916939908993858. [DOI] [PubMed] [Google Scholar]
- Co DO, Hogan LH, Kim SI, Sandor M. Mycobacterial granulomas: keys to a long-lasting host-pathogen relationship. Clin Immunol. 2004;113(2):130–136. doi: 10.1016/j.clim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Co DO, Hogan LH, Karman J, Heninger E, Vang S, Wells K, Kawaoka Y, Sandor M. Interactions between t cells responding to concurrent mycobacterial and influenza infections. J Immunol. 2006;177(12):8456–8465. doi: 10.4049/jimmunol.177.12.8456. [DOI] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5(10):975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. Ifn-gamma and no in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10(5):221–226. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. 2003;57:641–676. doi: 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding cd4 t cells during mycobacterial infection by inducing apoptosis of activated cd4 t cells. J Exp Med. 2000;192(1):117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to mycobacterium tuberculosis infection. Am J Pathol. 2004;164(1):49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10(9):958–64. doi: 10.1038/ni.1775. Erratum in: (2010) Nat Immunol 11(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of il-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target dc-sign to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson B, Zhong J, Cheers C. Anergy, ifn-gamma production, and apoptosis in terminal infection of mice with Mycobacterium avium. J Immunol. 1999;163(4):2073–2080. [PubMed] [Google Scholar]
- Gilleron M, Quesniaux VF, Puzo G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus calmette guerin and mycobacterium tuberculosis h37rv and its implication in toll-like receptor response. J Biol Chem. 2003;278(32):29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of il-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237(2):123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hogan LH, Macvilay K, Barger B, Co D, Malkovska I, Fennelly G, Sandor M. Mycobacterium bovis strain bacillus calmette-guerin-induced liver granulomas contain a diverse tcr repertoire, but a monoclonal t cell population is sufficient for protective granuloma formation. J Immunol. 2001;166(10):6367–6375. doi: 10.4049/jimmunol.166.10.6367. [DOI] [PubMed] [Google Scholar]
- Jo EK. Mycobacterial interaction with innate receptors: Tlrs, c-type lectins, and nlrs. Curr Opin Infect Dis. 2008;21(3):279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. Il-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting edge: regulatory t cells prevent efficient clearance of mycobacterium tuberculosis. J Immunol. 2007;178(5):2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. Tlr-activated b cells suppress t cell-mediated autoimmunity. J Immunol. 2008;180(7):4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- Lee J, Reinke EK, Zozulya AL, Sandor M, Fabry Z. Mycobacterium bovis bacille calmette-guerin infection in the CNS suppresses experimental autoimmune encephalomyelitis and th17 responses in an ifn-gamma-independent manner. J Immunol. 2008;181(9):6201–6212. doi: 10.4049/jimmunol.181.9.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Ben-Nun A. Bacterial agents protect against autoimmune disease. I. Mice pre-exposed to bordetella pertussis or mycobacterium tuberculosis are highly refractory to induction of experimental autoimmune encephalomyelitis. J Autoimmun. 1992;5 (6):675–690. doi: 10.1016/0896-8411(92)90185-s. [DOI] [PubMed] [Google Scholar]
- Li L, Lao SH, Wu CY. Increased frequency of cd4(+)cd25 (high) treg cells inhibit bcg-specific induction of ifn-gamma by cd4(+) t cells from tb patients. Tuberculosis (Edinb) 2007a;87(6):526–534. doi: 10.1016/j.tube.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Li X, McKinstry KK, Swain SL, Dalton DK. Ifn-gamma acts directly on activated cd4+ t cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J Immunol. 2007b;179(2):939–949. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of cd4+cd25+ t regulatory cells and il-10 via b7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178(6):3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- Martins TC, Aguas AP. Changes in B and T lymphocytes associated with mycobacteria-induced protection of NOD mice from diabetes. J Autoimmune. 1996;9(4):501–507. doi: 10.1006/jaut.1996.0067. [DOI] [PubMed] [Google Scholar]
- Mauri C, Ehrenstein MR. The ‘short’ history of regulatory b cells. Trends Immunol. 2008;29(1):34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing b cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. Nat Immunol. 2007;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The cd14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for toll-like receptors. J Immunol. 1999;163(12):6748–6755. [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48(3):594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates il-10-producing regulatory b cell subset characterized by cd1d upregulation. Immunity. 2002;16(2):219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit il-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166(12):7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- O’Connor RA, Wittmer S, Dalton DK. Infection-induced apoptosis deletes bystander cd4+ t cells: a mechanism for suppression of autoimmunity during bcg infection. J Autoimmun. 2005;24(2):93–100. doi: 10.1016/j.jaut.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of ifn-gamma-induced class ii transactivator expression by a 19-kda lipoprotein from mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171(1):175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- Paolillo A, Buzzi MG, Giugni E, Sabatini U, Bastianello S, Pozzilli C, Salvetti M, Ristori G. The effect of bacille calmette-guerin on the evolution of new enhancing lesions to hypointense t1 lesions in relapsing remitting ms. J Neurol. 2003;250 (2):247–248. doi: 10.1007/s00415-003-0967-6. [DOI] [PubMed] [Google Scholar]
- Parent ME, Siemiatycki J, Menzies R, Fritschi L, Colle E. Bacille calmette-guerin vaccination and incidence of iddm in montreal, canada. Diabetes Care. 1997;20(5):767–772. doi: 10.2337/diacare.20.5.767. [DOI] [PubMed] [Google Scholar]
- Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen esat-6 of mycobacterium tuberculosis and tlr2 inhibits tlr signaling in macrophages. Nat Immunol. 2007;8(6):610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- Peters W, Ernst JD. Mechanisms of cell recruitment in the immune response to mycobacterium tuberculosis. Microbes Infect. 2003;5(2):151–158. doi: 10.1016/s1286-4579(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Qin HY, Sadelain MW, Hitchon C, Lauzon J, Singh B. Complete freund’s adjuvant-induced t cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol. 1993;150(5):2072–2080. [PubMed] [Google Scholar]
- Qin HY, Chaturvedi P, Singh B. In vivo apoptosis of diabetogenic t cells in nod mice by ifn-gamma/tnf-alpha. Int Immunol. 2004;16(12):1723–1732. doi: 10.1093/intimm/dxh173. [DOI] [PubMed] [Google Scholar]
- Qin HY, Mukherjee R, Lee-Chan E, Ewen C, Bleackley RC, Singh B. A novel mechanism of regulatory t cell-mediated down-regulation of autoimmunity. Int Immunol. 2006;18(7):1001–1015. doi: 10.1093/intimm/dxl035. [DOI] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (tlr2)-dependent-positive and tlr2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172(7):4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Raupach B, Kaufmann SH. Immune responses to intracellular bacteria. Curr Opin Immunol. 2001;13(4):417–428. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for cd4+cd25+ t cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144(1):25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristori G, Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, Buttinelli C, Ruggieri S, Colonnese C, Pozzilli C, Salvetti M. Use of bacille calmette-guerin (bcg) in multiple sclerosis. Neurology. 1999;53(7):1588–1589. doi: 10.1212/wnl.53.7.1588. [DOI] [PubMed] [Google Scholar]
- Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20(3):353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25(3–4):237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory t cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sandor M, Weinstock JV, Wynn TA. Granulomas in schistosome and mycobacterial infections: a model of local immune responses. Trends Immunol. 2003;24(1):44–52. doi: 10.1016/s1471-4906(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Sanjeevi CB, Das AK, Shtauvere-Brameus A. Bcg vaccination and gad65 and ia-2 autoantibodies in autoimmune diabetes in southern india. Ann N Y Acad Sci. 2002;958:293–296. doi: 10.1111/j.1749-6632.2002.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of foxp3-expressing t regulatory cells during tuberculosis. J Exp Med. 2007;204(9):2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DL, Reinke EK, Hogan LH, Sandor M, Fabry Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol Lett. 2002;82(1–2):101–110. doi: 10.1016/s0165-2478(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Sewell DL, Reinke EK, Co DO, Hogan LH, Fritz RB, Sandor M, Fabry Z. Infection with Mycobacterium bovis bcg diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific t cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol. 2003;10(4):564–572. doi: 10.1128/CDLI.10.4.564-572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident V{delta}1 + gamma}{delta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178(7):4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291(5508):1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis Bacille Calmette–Guerin infection. J Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Visser L, Melief MJ, van Riel D, van Meurs M, Sick EA, Inamura S, Bajramovic JJ, Amor S, Hintzen RQ, Boven LA, ‘t Hart BA, Laman JD. Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates. Am J Pathol. 2006;169(5):1671–1685. doi: 10.2353/ajpath.2006.060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Zganiacz A, Wang J, Sharma SK. Enhanced protection against fatal mycobacterial infection in scid beige mice by reshaping innate immunity with ifn-gamma transgene. J Immunol. 2001;167(1):375–383. doi: 10.4049/jimmunol.167.1.375. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for il-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires il-17 receptor-dependent signals. Blood. 2007;109(9):3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12(3):308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed mycobacterium vaccae-induced allergen-specific regulatory t-cells. Nat Med. 2002;8(6):625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]