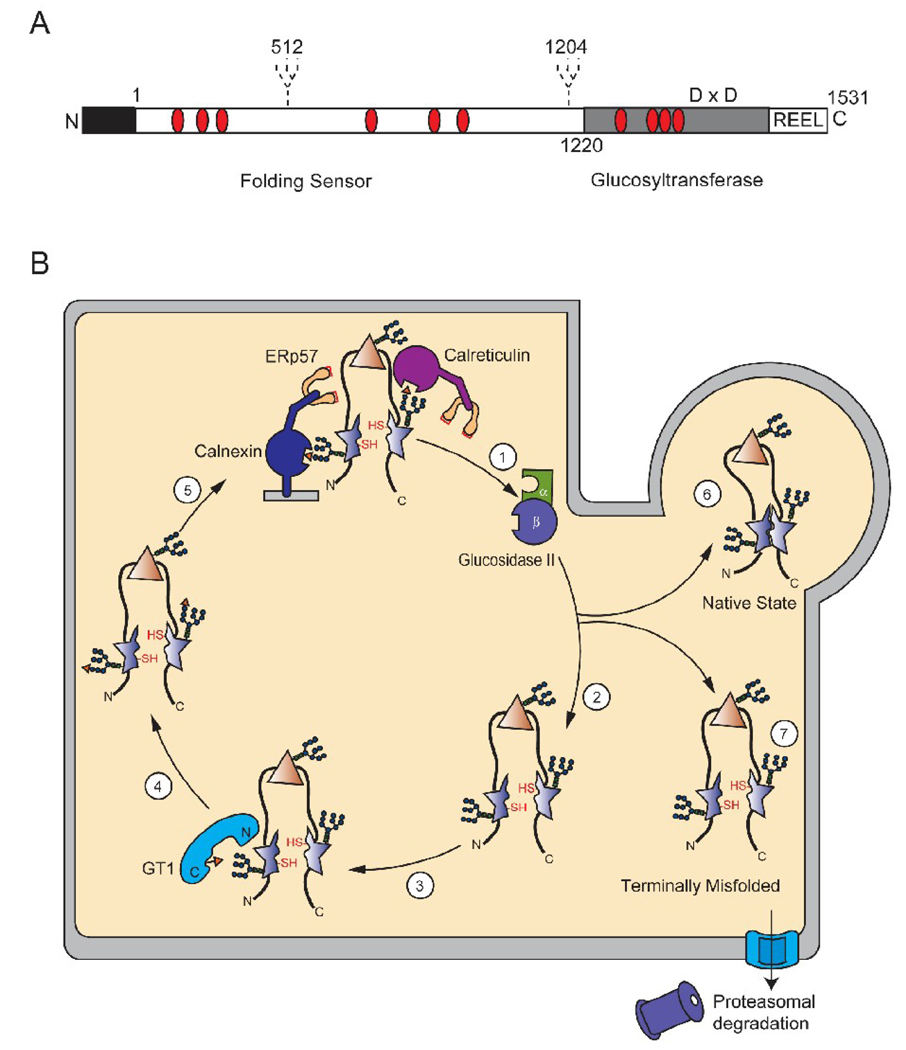
Figure 3.
Model of GT1 reglucosylation of a malformed glycoprotein. (A) Schematic representation of human GT1. Human GT1 is a 174-kDa protein with the mature protein consisting of 1531 amino acids after signal sequence cleavage. The N-terminus contains the protein recognition folding sensor domain, which represents 75% of the primary sequence and the cleavable signal sequence (black). The C-terminal domain contains the catalytic glucosyltransferase activity and the ER-retention signal R-E-E-L. In addition, the positioning of the two hypothetical glycosylation sites in human GT1 are depicted as dashed lines and Cys residues are marked with red ovals. The Asp-X-Asp motif is shown in the C-terminal domain (D × D) (B) Schematic of GT1-mediated reglucosylation in the ER. Glycoproteins are extracted from the lectin chaperone cycle by glucosidase II, which removes the last glucose in the A-branch (step 1). The chaperone released protein can attempt to fold to its native state, with certain domains being properly folded (triangle) and others being non-native or malformed (star) (step 2). GT1 can reglucosylate glycans situated near these non-native domains through docking to exposed hydrophobic patches (step 3). The substrate glycoprotein is now monoglucosylated (step 4). The newly reglucosylated protein can re-enter the calnexin and calreticulin binding cycle (step 5). The affinity of the lectin chaperones will decrease as the glycan is further trimmed by mannosidases and the protein will either be able to attain its native functional form (step 6) or be designated for degradation (step 7).