Abstract
The contribution of inflammation to hypertension and target organ damage is under investigation. The matrix metalloproteinase (MMP) enzymes are inflammatory mediators that may contribute to hypertension and its target organ consequences. Here we probe MMPs as inflammatory mediators in hypertension, by studying all three MMP classes in uncomplicated hypertension as well hypertension with profound renal damage, such as hypertensive end-stage renal disease (ESRD). We assayed plasma levels of five MMPs: one collagenase (MMP-1), two gelatinases (MMP-2, MMP-9), and two stromelysins (MMP-3, MMP-10). In hypertension, MMP-9 was elevated versus normotensive controls. Systolic blood pressure (SBP) in all three subject groups positively correlated with MMP-9. In hypertensive-ESRD, MMP-2 and MMP-10 were elevated compared to both hypertensive and normotensive subjects. Several correlations occurred across MMPs, suggesting coordinate biosynthetic control. Our results suggest discrete patterns of MMP overexpression in hypertension, with MMP-9 elevated early, and MMP-2 and MMP-10 linked to target organ damage.
Keywords: essential hypertension, end-stage renal disease (ESRD), matrix metalloproteinase (MMP), inflammation
Introduction
There is a growing body of evidence suggesting that chronic vascular inflammation plays a role in the development of essential hypertension, either as a pathogenic or secondary event (1). Inflammatory mediators, such as C-reactive protein (CRP), interleukin 1β (IL-1β); interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and reactive oxygen species (ROS), have been proposed to contribute to essential hypertension through several mechanisms, including enhancement of arterial stiffness and/or endothelial dysfunction (1). Long-term complications of hypertension include target organ damage, especially in the kidney. The mechanism whereby hypertension leads to chronic kidney disease and progression to end-stage renal disease (ESRD) remains poorly understood, and the extent to which inflammation contributes to this process is unknown.
Increased interest in inflammation has led to the investigation of additional inflammatory mediators, including the matrix metalloproteinase (MMP) family of enzymes. Matrix metalloproteinases are proteolytic enzymes that degrade the extracellular matrix (ECM), the basement membrane, and play a role in tissue repair and vascular remodeling (2, 3). At this point, 23 MMPs have been identified in humans, but substrate specificity for MMPs is not completely characterized. Known substrates include most of the ECM components (e.g., fibronectin, laminin) and many types of collegen (3). Matrix metalloproteinases can also cleave several classes of cell surface molecules, including adhesion molecules, receptors, chemokines, cytokines, growth factors, proteases, intercellular junction proteins, and structural molecules (4). Matrix metalloproteinase proteolysis of these cell surface molecules can result in maturation, degradation, activation, or inactivation of the molecules, leading to complex regulation of physiology by MMPs (4). For example, both MMP-2 and MMP-9 have pro-and anti-inflammatory effects (2). Matrix metalloproteinase have recently been shown to be associated with receptor cleavage of the insulin receptor leading to insulin resistance (5), a state typically associated with hypertension.
Matrix metalloproteinases have been implicated in the development and progression of many cardiovascular diseases, including essential hypertension (3). Elevation of plasma MMP-9 has been associated with increased arterial stiffness and elevated blood pressure in essential hypertension (6), but much of the existing data on plasma MMP-levels in hypertension is inconsistent. Other investigations have suggested that plasma MMP-9 is actually decreased in hypertensive subjects compared to normotensive controls (7, 8). In addition, a focused investigation of plasma MMPs in ESRD subjects with kidney failure attributed to essential hypertension (hypertensive-ESRD) has not been reported in the literature.
The most commonly studied MMPs in essential hypertension are MMP-2 and MMP-9. The purpose of this study is to broaden the scope of our understanding of MMP involvement in hypertension, by studying all three classes of soluble MMPs (collagenases, gelatinases, and stromelysins) in not only uncomplicated hypertension, but also in hypertension with severe target organ damage. To that end, we determined the levels of five MMPs (MMP-1, MMP-2, MMP-3, MMP-9, MMP-10) in three populations of human subjects: 1) normotensive controls; 2) essential hypertensive subjects; and 3) ESRD subjects with kidney failure attributed to essential hypertension. Levels of plasma MMP-3 and MMP-10 have not previously been reported in essential hypertension, and no plasma MMP data has been reported in a population of hypertensive-ESRD subjects.
Methods
Human Subjects
All participants gave written, informed consent. Plasma was isolated from antecubital venous blood collected onto heparin, and then frozen at −80°C prior to immunoassays in batches. Blood pressure was measured in seated subjects by an aneroid cuff sphygmomanometer. The normotensive control group consisted of 18 healthy volunteers with no history of hypertension. The hypertensive group consisted of 20 volunteers diagnosed with essential hypertension with initial criteria of SBP > 140 mmHg or DBP > 90 mmHg, or both; subsequently, each individual was treated with anti-hypertensive medications (ACEI in n = 9, alpha adrenergic antagonists in n = 6, angiotensin receptor blockers in n = 3, beta adrenergic antagonists in n = 11, calcium channel blockers in n = 4, and diuretics in n = 7, alone or in combination). The ESRD group consisted of 30 African-Americans on dialysis with kidney failure attributed to essential hypertension (severe hypertension with renal failure in the absence of diabetes or excessive proteinuria). Each ESRD subject was treated with anti-hypertensive medications (ACEI in n = 9, alpha adrenergic antagonists in n = 1, alpha adrenergic agonists in n = 6, angiotensin receptor blockers in n = 5, beta adrenergic antagonists in n = 20, calcium channel blockers in n = 15, and diuretics in n = 8, alone or in combination).
Matrix Metalloproteinase Immunoassays
The plasma concentrations of MMP-1, MMP-2, MMP-3, and MMP-10 were measured using the MS2400 Human MMP 2-Plex and 3-Plex Ultra-Sensitive Kits and the Sector Imager 2400 (Meso Scale Discovery, Gaithersburg, MD) based on electrochemiluminescence detection technology. Data reduction and calculation were performed with MSD Discovery workbench software (v 3.0.17.3). The concentration of MMP-9 was measured using R&D System’s ELISA Kit (Minneapolis, MN) on a Spectramax M5 plate reader equipped with SoftmaxPro v5.2 software for data reduction (Molecular Devices, Sunnyvale, CA). The intraassay coefficient of variation (CV) ranges were as follows: MMP-1: 2.1–12.9%; MMP-2: 1.1–10.8%; MMP-3: 2.5–11.6%; MMP-9: 0.9–7.4%; and MMP-10: 1.6–12.3%. The inter-assay CV ranges were as follows: MMP-1: 2.3–10.1%; MMP-2: 1.2–10.4%; MMP-3: 1.0–10.9%; MMP-9: 2.4–12.3%; and MMP-10: 0.9–9.5%.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). All statistical analyses, including univariate analysis of variance (ANOVA) with sex, age, and biogeographical ancestry as covariates, pairwise comparison of subject groups corrected for multiple comparisons, chi-square tests, and computation of partial correlation coefficients, were performed with SPSS version 11.5.0 for Windows (SPSS Inc., Chicago, IL).
Results
Subject Population Demographics and Clinical Characteristics
The mean age of the normotensive control group (34.7 ± 4.3 years) is significantly less than the mean age of the hypertensive (60.5 ± 2.7 years, p < 0.001) and ESRD groups (53.9 ± 2.3 years, p < 0.001) (Table 1). The normotensive group displayed a reduced body-mass index (BMI) (24.4 ± 1.4 kg/m2), systolic blood pressure (SBP) (122 ± 6 mm Hg), and diastolic blood pressure (DBP) (66 ± 3 mm Hg) compared to the hypertensive group (BMI: 30.1 ± 1.3 kg/m2, p = 0.008; SBP: 143 ± 5 mm Hg, p = 0.016; DBP: 81 ± 3 mm Hg, p = 0.006) (Table 1). The normotensive group displayed a reduced SBP, DBP, and HR compared to the ESRD group (SBP: 150 ± 4 mm Hg, p < 0.001; DBP: 81 ± 2 mm Hg, p = 0.001; heart rate (HR): 87 ± 2 beats/minute, p < 0.001) (Table 1). The distribution of biogeographical ancestry (chi-square test, p < 0.001) and sex (chi-square test, p < 0.001) differed significantly among the normotensive, hypertensive, and ESRD groups (Table 1). Age, sex, and biogeographical ancestry were therefore treated as co-variates in the statistical model and MMP levels were adjusted accordingly.
Table 1.
Demographic and clinical characteristics of the normotensive, hypertensive, and ESRD subjects. Numerical data was analyzed with univariate ANOVA followed by pairwise comparisons corrected for multiple comparisons. Categorical data (i.e., sex, biogeographical ancestry) was analyzed using chi-square tests. The physical and biochemical data are presented as age, sex, and biogeographical ancestry adjusted values. Data is presented as mean ± SEM
| n | Normotensive controls n = 18 | Essential hypertension n = 20 | Hypertensive-ESRD n = 30 | ANOVA/chi-square |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 34.7 ± 4.3 | 60.5 ± 2.7* | 53.9 ± 2.3* | p < 0.001 |
| Sex (male/female) | 9 (50%)/9 (50%) | 20 (100%)/0 (0%) | 23 (77%)/7 (23%) | p < 0.001 (chi square) |
| Anti-hypertensive medications (number/subject) | 0 ± 0 (0%) | 2.0 ± 0.3* (100%) | 2.4 ± 0.3* (100%) | p < 0.001 |
| Biogeographic ancestry | p < 0.001 (chi square) | |||
| Caucasian | 7 | 10 | 0 | |
| African American | 0 | 4 | 30 | |
| Hispanic | 5 | 3 | 0 | |
| Filipino | 1 | 1 | 0 | |
| East Asian | 3 | 0 | 0 | |
| South Asian | 1 | 0 | 0 | |
| Native American | 1 | 1 | 0 | |
| Other | 0 | 1 | 0 | |
| Physical | ||||
| SBP (mm Hg) | 122 ± 6 | 143 ± 5* | 150 ± 4* | p = 0.001 |
| DBP (mm Hg) | 66 ± 3 | 81 ± 3* | 81 ± 2* | p = 0.004 |
| HR (beats/min) | 66 ± 4 | 74 ± 3 | 87 ± 2*, # | p < 0.001 |
| BMI (kg/m2) | 24.4 ± 1.4 | 30.1 ± 1.3* | 27.9 ± 0.9 | p = 0.028 |
| Biochemical | ||||
| MMP-1 (ng/mL) | −1.8 ± 25.8 | 11.9 ± 23.4 | 41.9 ± 17.4 | p = 0.307 |
| MMP-2 (ng/mL) | 186.0 ± 22.9 | 165.5 ± 20.6 | 244.0 ± 15.6*,# | p = 0.006 |
| MMP-3 (ng/mL) | 30.4 ± 20.7 | 55.8 ± 18.8 | 72.8 ± 14.0 | p = 0.254 |
| MMP-9 (ng/mL) | 80.1 ± 20.3 | 146.4 ± 18.4* | 157.9 ± 13.6* | p = 0.013 |
| MMP-10 (ng/mL) | 2.4 ± 1.1 | 1.9 ± 1.0 | 6.6 ± 0.7*,# | p < 0.001 |
Significant at p < 0.05 compared to the normotensive group.
Significant at p < 0.05 compared to the hypertensive group.
MMP in Essential Hypertension
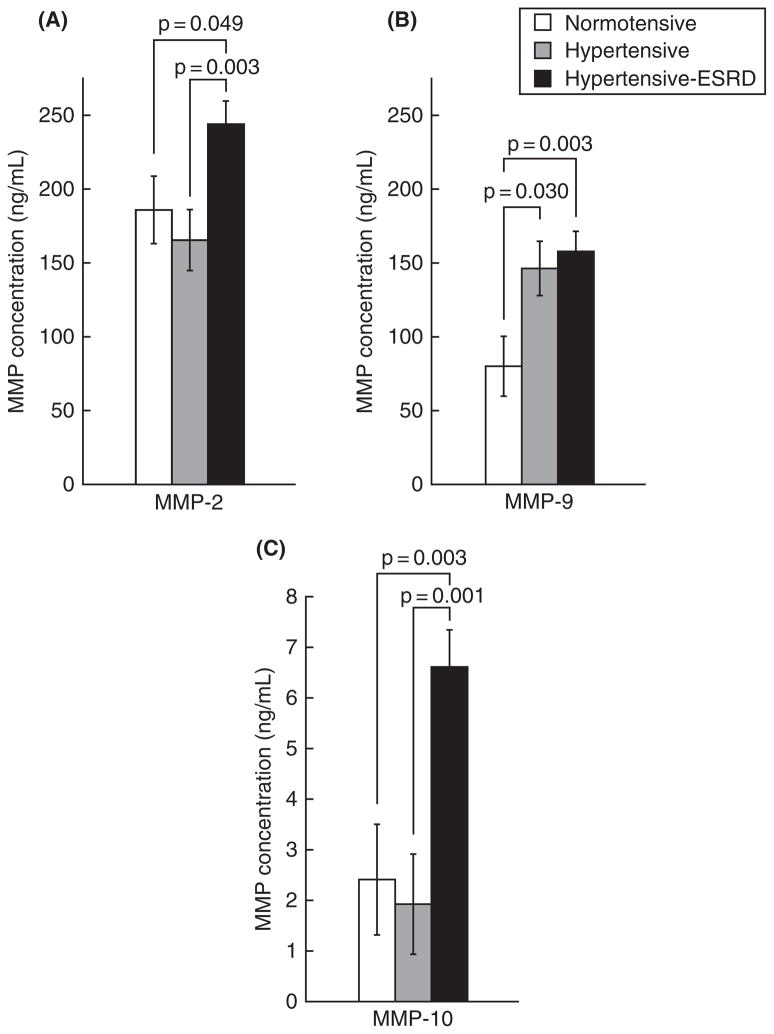
Elevated plasma levels of MMP-9 were observed in hypertensive subjects (146.4 ± 18.4 ng/mL) compared to normotensive subjects (80.1 ± 20.3 ng/mL; p = 0.030). (Table 1 and Figure 1). No differences in MMP-1, MMP-2, MMP-3, and MMP-10 were detected in the plasma of hypertensive subjects versus normotensive subjects.
Figure 1.
MMPs elevated in hypertension and hypertensive-ESRD. The levels of plasma MMP-2 (A), MMP-9 (B), and MMP-10 (C) are shown in normotensive, hypertensive, and hypertensive-ESRD subjects. Data was analyzed with ANOVA followed by pairwise comparisons corrected for multiple comparisons. P-values shown represent significance among the pairwise comparisons.
MMP in Hypertensive ESRD
Plasma MMP-9 was elevated in ESRD subjects (157.9 ± 13.6 ng/mL) compared to the normotensive subjects (80.1 ± 20.3 ng/mL; p = 0.003) (Table 1 and Figure 1). Plasma MMP-2 was elevated in ESRD subjects (244.0 ± 15.6 ng/mL) compared to both the hypertensive (165.5 ± 20.6 ng/mL; p = 0.003) and normotensive (186.0 ± 22.9 ng/mL; p = 0.049) subjects (Table 1 and Figure 1). Elevated plasma levels of MMP-10 were also observed in ESRD subjects (6.6 ± 0.7 ng/mL) compared to both the hypertensive (1.9 ± 1.0 ng/mL; p < 0.001) and normotensive (2.4 ± 1.1 ng/mL; p = 0.003) groups (Table 1 and Figure 1). No differences in plasma MMP-1 or MMP-3 were detected in the ESRD group compared to the hypertensive or normotensive groups.
MMPC
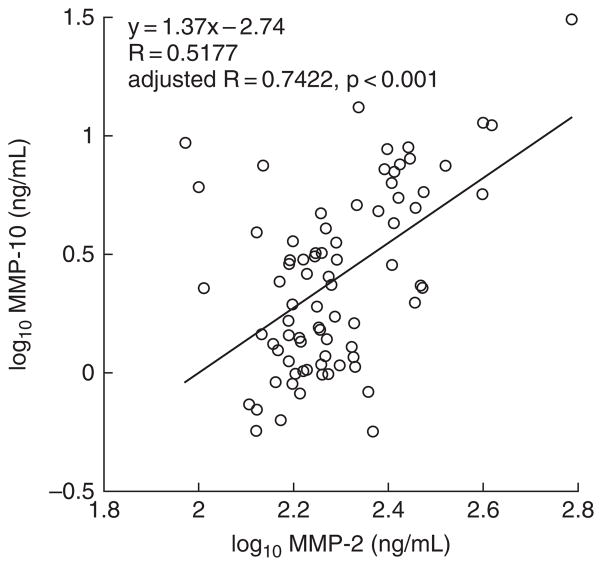
MMP-on-MMP partial correlation coefficients computed in the set of all three subject groups combined (i.e., normotensive, hypertensive, and ESRD) were controlled for age, sex, and biogeographical ancestry. Statistically significant (p < 0.05) positive correlations were found between MMP-1 and MMP-2 (R = 0.2786, p = 0.027), MMP-1 and MMP-9 (R = 0.3898, p = 0.002), MMP-1 and MMP-10 (R = 0.3480, p = 0.005), MMP-2 and MMP-3 (R = 0.2651, p = 0.036), and MMP-2 and MMP-10 (R = 0.7422, p < 0.001) (Table 2 and Figure 2).
Table 2.
MMP-on-MMP correlations in the normotensive, hypertensive, and ESRD subjects. The partial correlation coefficients (R) between MMPs controlled for age, sex, and biogeographical ancestry are presented
| MMP-1 | MMP-2 | MMP-3 | MMP-9 | MMP-10 | |
|---|---|---|---|---|---|
| MMP-1 | 1.0000 | 0.2786 | 0.0793 | 0.3898 | 0.3480 |
| MMP-2 | 1.0000 | 0.2651 | 0.1742 | 0.7422 | |
| MMP-3 | 1.0000 | 0.2264 | 0.1965 | ||
| MMP-9 | 1.0000 | 0.1491 | |||
| MMP-10 | 1.0000 |
Statistically significant correlation coefficients (at p < 0.05) are indicated in bold text.
Figure 2.
Correlation of plasma MMP-2 and MMP-10. Correlation analysis controlled for age, sex, and biogeographical ancestry revealed a statistically significant positive correlation (adjusted R = 0.7422, p < 0.001) between plasma MMP-2 and MMP-10 in the set of normotensive, hypertensive, and hypertensive-ESRD subjects. The log10 transformed values for unadjusted plasma MMP-2 and MMP-10 in each subject are displayed in the scatterplot.
MMP trait-on-trait (i.e., MMP on SBP, DBP, HR, and BMI) partial correlation coefficients computed in the set of all three subject groups combined (i.e., normotensive, hypertensive, and ESRD) were also controlled for age, sex, and biogeographical ancestry. Statistically significant (p < 0.05) correlations were found between MMP-9 and SBP (R = 0.2576, p = 0.042), and between MMP-2 and BMI (R = −0.3517, p = 0.005) (Table 3).
Table 3.
MMP trait-on-trait correlations in the normotensive, hypertensive, and ESRD subjects. The partial correlation coefficients (R) between MMPs and clinical phenotypes controlled for age, sex, and biogeographical ancestry are presented
| MMP-1 | MMP-2 | MMP-3 | MMP-9 | MMP-10 | |
|---|---|---|---|---|---|
| SBP | 0.2007 | −0.1254 | 0.1011 | 0.2576 | −0.0174 |
| DBP | 0.1408 | −0.1098 | −0.0605 | 0.1840 | −0.0787 |
| BMI | −0.0380 | −0.3517 | −0.1904 | −0.0389 | −0.1030 |
| HR | 0.1552 | 0.0082 | −0.0040 | 0.0027 | 0.2278 |
Statistically significant correlation coefficients (at p < 0.05) are indicated in bold text.
Discussion
Matrix metalloproteinases are implicated in the pathology of vascular diseases and several studies suggest that MMPs also play pathogenic roles in these disorders (3). For example, identification of a functional polymorphism in the promoter of the MMP-9 gene (9), which is associated with severity of arterial stiffness and hypertension (6), indicates that contributions of MMPs towards hypertension originate at the genetic level. In addition, the observation that plasma MMP-9 is elevated in healthy, normotensive subjects with high-normal blood pressure (10) (a pathological stage in the progression towards complete expression of essential hypertension) further supports a pathogenic role for MMP dysfunction in essential hypertension (10). However, the specific MMPs that are abnormal in essential hypertension and their relative expression levels in hypertensives and normotensive controls remains unclear. Here we explored the expression of a spectrum of MMPs in not only uncomplicated hypertension but also hypertension resulting in severe target organ (renal) damage.
MMPs and Essential Hypertension
We assayed a total of five MMP isoforms (MMP-1, MMP-2, MMP-3, MMP-9, and MMP-10), designed to span the major categories of soluble MMPs: collagenases (MMP-1), gelatinases (MMP-2, MMP-9), and stromelysins (MMP-10). We detected all five enzymes in the plasma of hypertensives and normotensive controls (Table 1). The most commonly studied MMPs in human essential hypertension are MMP-2 and MMP-9; to the best of our knowledge, we are the first to report MMP-3 and MMP-10 measurements in human essential hypertension. We observed that MMP-9 is present in significantly higher levels in essential hypertension than in normotensive controls (Figure 1), which is consistent with previous studies (11, 12). Elevation of MMP-9 is important because abnormal MMP levels can stimulate vascular inflammation, a potential contributor in the pathogenesis and progression of essential hypertension (1, 3). The pathogenic role of MMP-9 and inflammation in essential hypertension is further supported by the positive correlation of plasma MMP-9 levels with SBP (R = 0.2576, p = 0.042) (Table 3).
Previous studies, however, have also reported decreased MMP-9 levels in hypertension (7, 8) as well as no difference in MMP-9 between hypertensives and normotensive controls (13). We also observed no difference in plasma levels of MMP-1 or MMP-2, but previous studies have reported decreased levels of MMP-1 (14), decreased levels of MMP-2 (8), or increased levels of MMP-2 (12) in hypertensive cases versus normotensive controls. It is likely that inconsistencies in reported plasma MMP levels reflect the complex regulation of inflammation by MMPs, as well as differences in study design, subject populations, or statistical power. Indeed, MMP-2 and MMP-9 are known to have both pro- and anti-inflammatory effects (2), and several physiological parameters have been reported to affect plasma MMP levels: the presence and type of anti-hypertensive drug treatment (3, 8, 13), and the presence and severity of co-morbidities such as left-ventricular hypertrophy (3, 13) or end-organ damage (8). Of note, all hypertensive subjects were treated with angiotensin-converting enzyme (ACE) inhibitors (n = 9), alpha adrenergic antagonists (n = 6), angiotensin receptor blockers (n = 3), beta adrenergic antagonists (n = 11), calcium channel blockers (n = 4), and diuretics (n = 7), alone or in combination. ACE inhibitors have been demonstrated to inhibit MMP activity (15) and calcium channel blockers have been shown to increase (felodipine (8), amlodipine (16)) or have no effect on plasma MMP levels (diltiazem (8), amlodipine (16)). Common over-the-counter cycloxygenase inhibitors (e.g., aspirin) have also been shown to inhibit MMP activity (17, 18). Nine of the 20 hypertensive subjects reported use of cycloxygenase inhibitors (aspirin in n = 5; ibuprofen in n = 1; acetaminophen in n = 3). The effects of anti-inflammatory and anti-hypertensive medications are important in studies of MMPs, and even with a heterogenous medication profile in the hypertensive subjects, differences in plasma MMPs were detected.
Since essential hypertension is seldom an isolated disease, MMP levels reported by investigators could also differ if subject groups unknowingly exhibit proinflammatory or prothrombotic components of the metabolic syndrome (e.g., hyperglycemia, atherogenic dyslipidemia, elevated plasma fibrinogen). Indeed, mean BMI was elevated in our hypertensive subjects (Table 1). It has also been reported that plasma MMP levels vary in subtypes of essential hypertension: MMP-9 was found to be decreased in the plasma of salt-sensitive essential hypertension compared to salt-resistant essential hypertension (19). It is also possible that MMP inconsistencies in the literature stem from differences in sample size (i.e., statistical power), or the distribution of age, sex, or biogeographical ancestry of the subjects. In our subject population, age, sex, and biogeographical ancestry were unbalanced, though we accounted for such differences by co-variates in the statistical model; after such age-adjustment, BP status still had a significant effect upon plasma MMP-9 (Table 1 and Figure 1), nor did age alone predict plasma MMP concentrations (data not shown). It is well documented that age, sex, and biogeographical ancestry play a large role in hypertension susceptibility, but their influence on MMPs in health and essential hypertension is unclear. A hidden, systematic effect of age, sex, or biogeographical ancestry cannot be completely overlooked.
MMPs and End-Stage Renal Disease
Alteration of plasma MMPs in hypertensive ESRD has not previously been reported in the literature. All subjects in our ESRD sample population developed kidney failure as a complication of severe essential hypertension. For reasons that remain incompletely understood, hypertensive ESRD occurs most frequently in African-Americans, and this skewed distribution among biogeographical ancestries is reflected in our ESRD subject sample, i.e., the ESRD group is 100% African-American. Comparison of the ESRD group with the hypertensive group will help determine if MMPs contribute to hypertensive kidney failure in African-Americans.
We observed that both MMP-2 and MMP-10 are elevated in ESRD compared to both the normotensive and hypertensive groups, but that MMP-2 and MMP-10 do not differ between the normotensive and hypertensive groups (Table 1 and Figure 1). This suggests that MMP-2 and MMP-10 do not play a major role in essential hypertension but instead may be involved in the development of renal injury once essential hypertension has been established. Interestingly, MMP-2 and MMP-10 have a strong positive correlation (R = 0.7422, p < 0.001) (Figure 2), which suggests co-regulation of MMPs in effecting end-organ damage in hypertension. Elevation of MMP-2 and MMP-10 could also result from impaired filtration in the damaged kidneys, but this seems unlikely since the relatively large size of MMPs (~60 kDa) would apparently preclude their excretion from the kidneys in either health or disease; furthermore, simple attribution of elevated MMP-2 and -10 concentrations to diminished glomerular filtration in ESRD would not account for the unremarkable levels of the other MMPs (MMP-1, MMP-3) in ESRD.
We also determined that MMP-9 is elevated in both hypertensive and ESRD groups compared to the normotensive group (Table 1 and Figure 1), but that MMP-9 does not differ between hypertensive and ESRD groups (Table 1 and Figure 1). This suggests that MMP-9 is involved in essential hypertension, but it is unclear as to whether MMP-9 contributes to ESRD in addition to hypertension or if elevated MMP-9 simply reflects the hypertensive status of ESRD subjects.
It is also possible that elevations of MMP-2, MMP-9, and MMP-10 in ESRD result, in part, from complications of hemodialysis, including inflammation. However, a previous study compared plasma MMP levels in subjects (with diabetes mellitus or chronic glomerulonephritis) before and after dialysis and found that MMP-2 and MMP-9 were actually reduced after dialysis (20). While interesting, this result only addresses the effect of acute dialysis on plasma MMP levels. Further research is required to understand the effect of long-term dialysis on plasma MMPs. Another study examined plasma MMP levels in hemodialysis subjects (with ESRD attributed to glomerulonephritis, interstitial nephritis, polycystic kidney disease, and unknown causes in several cases) with and without cardiovascular disease (hypertension, ischemic heart disease, and/or peripheral vascular disease); it was determined that plasma MMP-2 was increased in hemodialysis subjects with or without cardiovascular disease, as compared to healthy controls (21). No change was reported in plasma MMP-9 levels in hemodialysis subjects with or without cardiovascular disease compared to the healthy control population (21). While the above evidence suggests that dialysis per se can modulate plasma MMP levels, additional studies are needed to isolate and identify the individual effects of dialysis, ESRD, and the underlying causal diseases. In our study, we have two variables that can affect plasma MMP levels: hypertension (disease) and hypertensive-ESRD (kidney failure). We cannot completely isolate the individual effects of hypertension and hypertensive-ESRD on plasma MMP levels. A normotensive ESRD group could provide additional information about the individual contributions of hypertension and ESRD; however, the underlying causal diseases of kidney failure in a normotensive ESRD group can vary widely, and might add additional complexity to the interpretation.
Since our ESRD population consisted of only African-Americans, further research may be required to understand the effects of biogeographical ancestry on plasma MMPs. It is important to note that biogeographical ancestry does, in fact, play a major role in susceptibility to hypertension and hypertensive-ESRD. African-Americans have a higher prevalence of essential hypertension than other racial and ethnic groups. African-Americans also have a substantially higher rate of progression from essential hypertension to ESRD than other racial and ethnic groups. It is possible that MMPs contribute to these effects, but racial and ethnic differences in MMP expression are poorly understood.
MMP Correlations
Partial correlation coefficients (controlled for age, sex, and biogeographical ancestry) were computed in the set of all three subject groups combined (normotensive, hypertensive, and ESRD). Five statistically significant, positive correlations were identified between the MMPs ranging from R = 0.2651 to R = 0.7422. These correlations suggest co-regulation of MMPs and their involvement in common molecular mechanisms of inflammation. Interestingly, the highest MMP-on-MMP correlation coefficient (R = 0.7422) was between MMP-2 and MMP-10, the only two MMPs elevated solely in ESRD. Whether MMP-2 and MMP-10 act coordinately to cause kidney damage in hypertension is currently unexplored.
Computation of MMP trait-on-trait (i.e., MMP on SBP, DBP, HR, and BMI) correlations revealed two significant relationships. MMP-9 is positively correlated with SBP (R = 0.2576, p = 0.042), which parallels our observation of elevated MMP-9 in hypertension (Table 1), and further suggests that MMP-9 contributes to the trait. The other relationship found was a negative correlation of MMP-2 with BMI (R = −0.3517, p = 0.005). End-stage renal disease subjects frequently experience reductions in BMI, as a consequence of impaired appetite. Indeed, the mean BMI is less in ESRD subjects than in hypertensive subjects, though the difference does not reach statistical significance (p = 0.053). The negative correlation between MMP-2 and BMI is likely driven by elevated plasma MMP-2 in ESRD subjects that exhibit reductions in BMI in parallel with hypertensive disease progression. In fact, MMP-2 and BMI lack a statistically significant correlation among only the normotensive and hypertensive subjects (R = −0.202, p = 0.251).
Conclusions
We observed elevation of plasma MMP-9 in essential hypertension and a positive correlation between plasma MMP-9 and SBP. We also observed elevation of plasma MMP-2 and MMP-10 in subjects with hypertensive-ESRD and a positive correlation between plasma MMP-2 and plasma MMP-10 levels. Matrix Metallo proteinases are candidates for pathological molecular mechanisms in essential hypertension and hypertensive-ESRD, since the enzymes degrade the extracellular-matrix, regulate tissue remodeling, and modulate vascular inflammation. At this point, the cause of plasma MMP variation in hypertension and hypertension-ESRD remains unknown and tissue MMP measurements in humans are clearly problematic. Thoughtful design and analysis of experiments is crucial to interpreting plasma MMP studies since subject population demographics, medications, and co-morbidities can influence circulating MMP levels. Studies are also needed to assess the relationship between plasma MMP levels and plasma MMP activity, since enzyme concentrations might change to compensate for irregularities in enzyme activity and vice versa. Further investigation is required to understand MMP abnormalities in essential hypertension and hypertensive-ESRD in distinct subpopulations that vary by biogeographical ancestry, sex, co-morbidity, and underlying causal disease.
Acknowledgments
These studies were carried out in part in the General Clinical Research Center, University of California, San Diego, with funding provided by the National Center for Research Resources, M01RR 000827, the Comprehensive Research Center of Excellence in Minority Health and Health Disparities (CRCHD), MD00020, and the NIH/NIDDK DK007671 Nephrology Training Grant, National Institutes of Health.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12:1623–1635. doi: 10.2174/138161206776843313. [DOI] [PubMed] [Google Scholar]
- 2.Ganea E, Trifan M, Laslo AC, Putina G, Cristescu C. Matrix metalloproteinases: useful and deleterious. Biochem Soc Trans. 2007;35:689–691. doi: 10.1042/BST0350689. [DOI] [PubMed] [Google Scholar]
- 3.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 5.DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: Mechanism for insulin resistance in spontaneously hypertensive rats. Hypertension. 2008;52:415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, Feely J, Spiers JP, Mahmud A. Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. J Hum Hypertens. 2007;21:861–867. doi: 10.1038/sj.jhh.1002244. [DOI] [PubMed] [Google Scholar]
- 7.Li-Saw-Hee FL, Edmunds E, Blann AD, Beevers DG, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy. Int J Cardiol. 2000;75:43–47. doi: 10.1016/s0167-5273(00)00274-6. [DOI] [PubMed] [Google Scholar]
- 8.Zervoudaki A, Economou E, Pitsavos C, Vasiliadou K, Aggeli C, Tsioufis K, Toutouza M, Stefanadis C, Toutouzas P. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and -9 in essential hypertension. Am J Hypertens. 2004;7:273–276. doi: 10.1016/j.amjhyper.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99:1788–1794. doi: 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos DP, Makris TK, Krespi PG, Poulakou M, Papazachou OG, Hatzizacharias AN, Perrea D, Votteas V. Changes in metalloproteinases in healthy normotensive patients with high-normal blood pressure. Eur Cytokine Netw. 2005;16:211–214. [PubMed] [Google Scholar]
- 11.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30:959–963. doi: 10.1291/hypres.30.959. [DOI] [PubMed] [Google Scholar]
- 12.Derosa G, D’Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Tinelli C, Cicero AF. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13:227–231. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: Relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 14.Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–540. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Han HC, Lindsey ML. ACE inhibitors to block MMP-9 activity: New functions for old inhibitors. J Mol Cell Cardiol. 2007;43:664–666. doi: 10.1016/j.yjmcc.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zervoudaki A, Economou E, Stefanadis C, Pitsavos C, Tsioufis K, Aggeli C, Vasiliadou K, Toutouza M, Toutouzas P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17:119–124. doi: 10.1038/sj.jhh.1001518. [DOI] [PubMed] [Google Scholar]
- 17.Jiang MC, Liao CF, Lee PH. Aspirin inhibits matrix metalloproteinase-2 activity, increases E-cadherin production, and inhibits in vitro invasion of tumor cells. Biochem Biophys Res Commun. 2001;282:671–677. doi: 10.1006/bbrc.2001.4637. [DOI] [PubMed] [Google Scholar]
- 18.Cipollone F, Fazia ML, Mezzetti A. Role of angiotensin II receptor blockers in atherosclerotic plaque stability. Expert Opin Pharmacother. 2006;7:277–285. doi: 10.1517/14656566.7.3.277. [DOI] [PubMed] [Google Scholar]
- 19.Larrousse M, Bragulat E, Segarra M, Sierra C, Coca A, de La Sierra A. Increased levels of atherosclerosis markers in salt-sensitive hypertension. Am J Hypertens. 2006;19:87–93. doi: 10.1016/j.amjhyper.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Chou FP, Chu SC, Cheng MC, Yang SF, Cheung WN, Chiou HL, Hsieh YS. Effect of hemodialysis on the plasma level of type IV collagenases and their inhibitors. Clin Biochem. 2002;35:383–388. doi: 10.1016/s0009-9120(02)00331-4. [DOI] [PubMed] [Google Scholar]
- 21.Pawlak K, Pawlak D, Mysliwiec M. Serum matrix metalloproteinase-2 and increased oxidative stress are associated with carotid atherosclerosis in hemodialyzed patients. Atherosclerosis. 2007;190:199–204. doi: 10.1016/j.atherosclerosis.2006.01.020. [DOI] [PubMed] [Google Scholar]