Abstract
Background: The purpose was to examine the prognostic impact of features of tumor cells and immune microenvironment in patients with follicular lymphoma treated with and without anti-CD20 monoclonal antibody therapy.
Patients and methods: Tissue microarrays were constructed from archived tissue obtained from patients on three sequential Southwest Oncology Group (SWOG) trials for FL. All three trials included anthracycline-based chemotherapy. Anti-CD20 monoclonal antibodies were included for patients in the latter two trials. Immunohistochemistry was used to study the number and distribution of cells staining for forkhead box protein P3 (FOXP3) and lymphoma-associated macrophages (LAMs) and the number of lymphoma cells staining for myeloma-associated antigen-1 (MUM-1). Cox proportional hazards regression was used to evaluate the association between marker expression and overall survival (OS).
Results: The number or pattern of infiltrating FOXP3 cells and LAMs did not correlate with OS in sequential SWOG studies for FL. The presence of MUM-1 correlated with lower OS for patients who received monoclonal antibody but not for those treated with chemotherapy alone.
Conclusions: Immune cell composition of lymph nodes did not correlate with OS in this analysis of trials in FL. The mechanism of the observed correlation between MUM-1 expression and adverse prognosis in patients receiving monoclonal antibody therapy requires confirmation.
Keywords: follicular lymphoma, LAMs, MUM-1, prognosis, Tregs
introduction
The clinical behavior of follicular lymphoma (FL) is variable. Clinical prognostic factors specific to FL have been described in the follicular lymphoma international prognostic index (FLIPI) [1]. This index is used widely in risk stratification for clinical trials. However, within each risk group defined by the FLIPI there is variability in outcome, reflecting the underlying biological heterogeneity of this disease. Identification of biological risk factors may add to the predictive value of the FLIPI and lead to the identification of new therapeutic targets.
The introduction of monoclonal antibody therapy has had a major impact on the management of FL. Prospective randomized studies in the first-line and relapsed settings have demonstrated improved progression-free and overall survival (OS) rates for patients treated with chemotherapy in combination with rituximab compared with chemotherapy alone [2–4]. In addition, population-based cooperative group and single-center studies have all reported recent improvements in OS for patients with FL, indicating that monoclonal antibody therapy may modify the clinical behavior of this disease [5–7].
Gene expression profiling (GEP) studies have provided an insight into the pathobiology of FL and indicated that characteristics of the host immune response are of particular importance [8]. Gene signatures characteristic of T-cell or macrophage host immune responses were in fact highly predictive of outcome. Subsequent studies have thus focused on the potential prognostic importance of T-cell subsets and macrophage infiltration in FL using immunohistochemistry-based techniques, most commonly as tissue microarrays (TMAs) [9–16]. These studies have produced conflicting results with respect to the prognostic impact of CD68+ lymphoma-associated macrophage (LAM), tumor-infiltrating lymphocytes, and the number and patterns of infiltration with regulatory T cells (Tregs) characterized by expression of forkhead box protein P3, FOXP3. Recent data reported by one of the authors indicate that expression of the multiple myeloma-associated antigen-1/interferon regulatory factor 4 (MUM-1/IRF4) may have clinical significance in FL [17].
The prognostic significance of these biomarkers has been variable, with discrepant results perhaps due to technical factors and/or differences in patient populations. One factor that may be of prime importance is the inclusion of monoclonal antibodies in some studies but not in others. The inclusion of rituximab has apparently overcome the adverse prognostic effect of some clinical factors. Indeed, recent studies in diffuse large B-cell lymphoma (DLBCL) have also shown that biological prognostic factors may be highly treatment dependent. These studies have indicated that the addition of rituximab to standard chemotherapy for advanced DLBCL appears to modify the prognostic impact of bcl-2 and bcl-6 expression [18, 19]. It is possible that this might also be true for patients with FL [16].
Further studies of biological prognostic factors in low-grade FL are therefore required in patients who have been consistently staged and treated, with prolonged follow-up, and including patients treated before and after the introduction of rituximab, to assess the impact of this and other monoclonal antibodies. We therefore examined the prognostic significance of LAMs, FOXP3-positive Tregs, and expression of MUM-1 in patients with FL treated on three Southwest Oncology Group (SWOG) protocols, including chemotherapy with no monoclonal antibody (S8809) [20], with rituximab (S9800) [21], and with the anti-CD20 radioimmunoconjugate I 131-tositumomab (S9911) [22].
patients and methods
selection of trials
Sequential trials conducted by SWOG for patients with previously untreated, advanced FL were identified, which spanned the period during which anti-CD20-directed monoclonal antibody therapy was introduced and for which archival tissue blocks were available. The studies identified were S8809, S9800, and S9911. Results of each of these studies have been published previously. All studies were conducted with the approval of local institutional review boards.
S8809 was a randomized trial in which 571 patients with advanced FL were treated initially with six to eight cycles of combination chemotherapy with ProMACE–MOPP (prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, procarbazine, and prednisone) [20]. Patients who responded were randomly assigned to observation alone, or interferon α2b consolidation, given three times per week for 2 years. With a median follow-up of 15.6 years, the 5-year progression-free survival and OS rates were 43% and 73%, respectively. No differences in overall or progression-free survival were observed according to the randomized arm.
S9800 was a phase II study in which 85 eligible patients with previously untreated advanced FL were treated with six cycles of combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) followed by rituximab, given at 1-week intervals for 4 weeks [21]. With a median follow-up of 9.6 years, the 5-year progression-free survival and OS rates were 46% and 88%, respectively.
S9911 was a phase II study in which 90 previously untreated patients with advanced FL were treated with six cycles of CHOP followed 4–8 weeks later by anti-CD20-directed radioimmunotherapy with tositumomab/I 131-tositumomab [22]. The overall response rate to this regimen was 90% [67% complete response (complete response is defined as complete disappearance of all previously documented disease)]. With a median follow-up of 8.0 years, the 5-year progression-free survival and OS rates were 68% and 88%, respectively.
patient selection
Archived tissue blocks were identified from the SWOG lymphoma repository. Tissue blocks were available from 103 patients treated on protocol S8809, 30 patients on S9800, and 47 patients on protocol S9911. Four of the 30 patients from the S9800 and 5 of 47 from the S9911 study did not receive monoclonal antibody on protocol, primarily because they failed to achieve an adequate response to initial CHOP. These patients have been included in the final analysis to avoid potential bias introduced by excluding patients who did not respond to chemotherapy, particularly since it is possible that these patients subsequently received monoclonal antibody therapy off protocol. Exclusion of these patients from the analysis did not significantly affect the results of the study for any of the markers.
TMA construction
After identification of cases, an initial histologic review was undertaken for confirmation of diagnosis and to assess adequacy of tissue for microarray construction. All cases were classified according to the World Health Organization classification [23].
For each case, 1-mm cores were prepared in triplicate. Immunohistochemistry was carried out using an automated immunostainer (Ventana Medical Systems, Tucson, AZ) and monoclonal antibodies to CD68 (PGM1; DAKO, Carpinteria, CA), MUM-1 (Mum1p; DAKO), and FOXP3 (clone 22510; Abcam, Cambridge, MA). No other immunohistochemical studies were carried out. The number of cells and patterns of infiltration were assessed. Tregs (FOXP3) were scored as positive cells per 5 high-power fields (5 hpf) and according to pattern (follicular/perifollicular versus evenly distributed) as previously described [17]. LAMs (CD68) were scored as intrafollicular (IF) LAMs per 5 hpf and extrafollicular (EF) LAMs per 5 hpf as previously described [17]. An hpf for the purposes of this study was a ×1000 field (Olympus BX41, 22-mm-diameter ocular; Olympus America, Center Valley, PA). MUM-1-positive cells were scored according to percentage of tumor cells with 20% as the cut-off. MUM-1-positive cells were generally within follicles and care was taken to ensure that the cells did not represent IF plasma cells. All staining was carried out in the laboratories of the Department of Anatomic Pathology at the Cleveland Clinic and interpreted by one of us (EDH). Qualitative reproducibility was determined as part of standard laboratory assay validation and controlled with each run.
Reproducibility of CD68 and FOXP3 quantitative scoring was tested by random blinded recounting of 20 cases for CD68 and 15 cases for FOXP3 on separate days. Pearson correlation (R) was 0.90 for EF counts and 0.82 for IF counts (P < 0.0001). The correlation for FOXP3 was 0.94 (P < 0.00001).
survival and statistical analysis
Associations between various features of marker expression and OS were evaluated by Cox proportional hazards regression. All analyses were stratified by chemotherapy alone (S8809) versus chemotherapy plus monoclonal antibody (S9800/S9911). Due to the sample size limitations, the primary analyses consist of univariate models. Multivariate models adjusting for age and lactate dehydrogenase (LDH) were also explored. To explore whether prognostic effect of any marker differed by whether monoclonal antibody therapy was given, interaction terms were added to these models, and they were also fit separately within each treatment stratum.
results
patient characteristics
Characteristics for eligible patients with tissue available for microarray construction are shown in Table 1. With respect to demographics, risk factors, and survival, patients with available tissue were generally similar across the three studies, although those from S9800 were somewhat older, those from S9911 had a lower frequency of elevated LDH, and those from S8809 had slightly worse survival. The distribution of international prognostic index (IPI) risk factors was similar across all three studies. Since not all FLIPI risk factors were available for S9800, we used the IPI [24] risk factors, which have been shown previously to have prognostic value in FL [25]. For S8809, the distribution of FLIPI scores was 0: 36%, 1: 38%, and 2: 26%. For S9911, the percentages were 36%, 45%, and 19%, respectively. For none of the three studies did the baseline characteristics of the subset of patients with tissue available differ substantially from those of the study population as a whole. However, survival was better in this subset of S8809 patients (5-year OS = 80% versus 69% among those excluded).
Table 1.
Characteristics of patients with tissue available for microarray construction
| S8809 (N = 103) | S9800 (N = 30) | S9911 (N = 47) | |
| Male | 55 (53%) | 19 (63%) | 28 (60%) |
| Caucasian | 92 (89%) | 30 (100%) | 45 (96%) |
| Median age (range) | 47.4 (26.1–69.4) | 54.8 (27.8–75.5) | 49.9 (22.9–66.8) |
| Follicular grade | |||
| 1 | 48 (47%) | 12 (40%) | 22 (46%) |
| 2 | 34 (33%) | 15 (50%) | 15 (32%) |
| 3a | 14 (13%) | 2 (7%) | 5 (11%) |
| 3b | 0 (0%) | 1 (3%) | 0 (0%) |
| Missing | 7 (7%) | 0 (0%) | 5 (11%) |
| IPI score | |||
| 0 | 61 (59%) | 15 (50%) | 27 (58%) |
| 1 | 34 (33%) | 11 (37%) | 19 (40%) |
| 2/3 | 8 (8%) | 4 (13%) | 1 (2%) |
| IPI risk factors | |||
| Performance status >1 | 1 (1%) | 1 (3%) | 0 (0%) |
| >1 Extranodal sites | 15 (15%) | 4 (13%) | 13 (28%) |
| Lactate dehydrogenase > upper limit of normal | 28 (27%) | 9 (30%) | 5 (11%) |
| Age >60 | 7 (7%) | 9 (30%) | 6 (13%) |
| Stage 3 or greater | 102 (99%) | 29 (97%) | 42 (89%) |
| Randomized | 56 (54%) | N/A | N/A |
| Interferon arm | 34 (61% of 56) | N/A | N/A |
| 5-Year overall survival (%) | 80 | 83 | 85 |
IPI, international prognostic index.
results of immunostaining
Results of immunostaining according to each study and for the entire patient population are shown in Table 2 and illustrated in Figure 1. For technical reasons, not all data were available for each case. MUM-1 data were available for 180 patients, FOXP3 counts were available for 152 patients, CD68 EF counts in 144 patients, and CD68 IF counts in 138 patients. For FOXP3 staining, the median positive cells per 5 hpf were 169 [interquartile range (IQR) 106–253]. A follicular or perifollicular pattern of infiltration was seen in 19.3% cases. For IF and EF LAMs, the median counts (IQR) were 68 (55–84) and 146 (120–188), respectively. Positive MUM-1 expression (defined as ≥20% tumor cells with staining) was seen in 13.9% of cases. Biomarker distributions across the three studies were similar. No differences were observed in biomarker distribution between patients in the S8809 and S9911 studies. Although patients in the S9800 study had higher FOXP3 and LAM counts than the other study populations, in light of the small sample size, these differences could be due to chance.
Table 2.
Results of immunostaining
| S8809 | S9800 | S9911 | Overall | |
| MUM-1, % 20%+ | 15.5 (N = 103) | 13.3 (N = 30) | 10.6 (N = 47) | 13.9 (N = 180) |
| FOXP3, % uniform distributiona | 81.3 (N = 80) | 75.0 (N = 28) | 83.3 (N = 42) | 80.7 (N = 150) |
| FOXP3 count median (IQR) | 169 (94–277) (N = 82) | 200 (137–263) (N = 28) | 156 (113–205) (N = 42) | 169 (106–253) (N = 152) |
| CD68EF count median (IQR) | 126 (103–153) (N = 68) | 246 (189–293) (N = 29) | 141 (125–178) (N = 47) | 146 (120–188) (N = 144) |
| CD68IF count median (IQR) | 70 (58–82) (N = 64) | 88 (69–108) (N = 28) | 57 (50–68) (N = 46) | 68 (55–84) (N = 138) |
Uniform distribution versus presence of perifollicular/follicular pattern.
MUM-1, myeloma-associated antigen-1; FOXP3, forkhead box protein P3; and IQR, interquartile range.
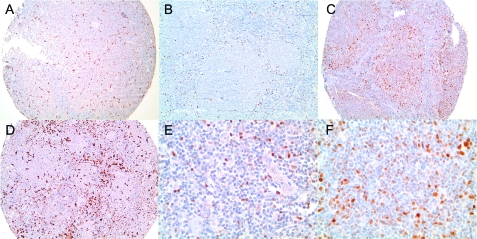
Figure 1.
Examples of immunostaining. (A,D) CD68 stain showing low and high numbers of macrophages, respectively. (B,E) Forkhead box protein staining shows a perifollicular pattern (B). High magnification shows nuclear expression (E). (C,F) A case showing expression of myeloma-associated antigen-1 at low (C) and high (F) magnifications. For all images, low magnification = ×100, whereas high magnification = ×400 (original magnification).
survival analysis
The results of univariate analysis of the association between OS and biomarker levels and patterns of staining are summarized in Table 3. Results are presented for LAM and FOXP3 counts dichotomized at the median levels given in Table 2. Other cut-off levels were also explored, including the previously published threshold of 17 hpf for EF CD68 [17]. Finally, all biomarker levels were also analyzed as continuous variables. In none of these analyses was a significant association observed between OS and either numbers or patterns of LAM or FOXP3 staining, either in the entire patient cohort or within either treatment stratum.
Table 3.
Hazard ratios for overall survival according to immunohistochemistry
| S8809 | S9800/S9911 (stratified by study) | Overall (stratified by study) | |
| MUM-1: 20%+ versus <20% | 1.21 (0.57, 2.59) | 4.85 (1.71, 13.81) | 1.75 (0.95, 3.22)a |
| FOXP3: uniform distribution versus other | 0.72 (0.33, 1.59) | 1.74 (0.39, 7.75) | 0.91 (0.46, 1.83) |
| FOXP3: above median versus below | 1.12 (0.58, 2.16) | 1.10 (0.37, 3.24) | 1.12 (0.64, 1.95) |
| CD68EF: above median versus below | 0.88 (0.40, 1.93) | 0.39 (0.10, 1.48) | 0.71 (0.35, 1.43) |
| CD68IF: above median versus below | 0.74 (0.35, 1.58) | 1.43 (0.43, 4.80) | 0.90 (0.47, 1.70) |
Test for interaction between MUM-1 expression and treatment stratum significant at P = 0.04.
MUM-1, myeloma-associated antigen-1; FOXP3, forkhead box protein P3.
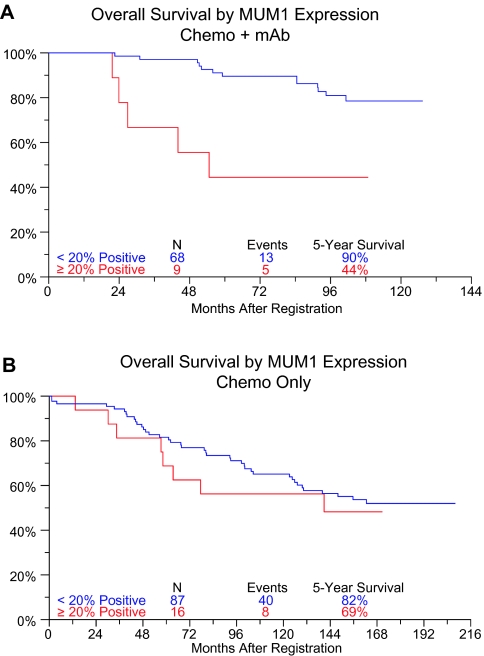
As shown in Table 3, in the entire dataset, elevated MUM-1 expression was associated with a nearly twofold increase in the risk of death [hazard ratio (HR) = 1.75, 95% confidence interval (CI) 0.95–3.22, P = 0.07]. The test for interaction between monoclonal antibody therapy and MUM-1 expression was also significant (P = 0.04), indicating that the effect of MUM-1 expression on survival is modified by monoclonal antibody therapy. Within S8809 patients (chemotherapy only), MUM-1 expression was not associated with survival [HR (95% CI) = 1.21 (0.57, 2.59), P = 0.62]. Within S9800/S9911 patients (chemotherapy plus monoclonal antibody therapy), however, elevated MUM-1 expression was associated with a nearly fivefold increase in the risk of death [HR (95% CI) = 4.85 (1.71, 13.81), P = 0.0031] (Figure 2). No significant association between MUM-1 pattern and survival was observed. In multivariate models adjusting for age and LDH, all results were similar.
Figure 2.
Overall survival according to myeloma-associated antigen-1 expression for patients receiving chemotherapy plus monoclonal antibody on studies S9800 and S9911 (A) and chemotherapy only on study S8809 (B).
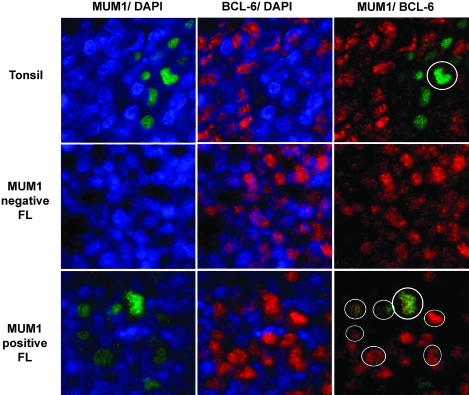
Since previously published studies indicated that MUM-1 was not expressed in FL [23], we used immunohistochemistry to identify several additional MUM-1-expressing FL cases from the Cleveland Clinic that had not been included in the prior analysis. A dual-color quantum dot immunofluorescent assay was then carried out to assess bcl-6 and MUM-1 expression simultaneously. Staining of hyperplastic lymphoid follicle centers confirmed that <2% of bcl-6+ cells coexpressed MUM-1, consistent with the mutually exclusive expression of these two transcription factors in normal B-cell development. However, the three MUM-1-positive FL cases tested each showed clear coexpression of nuclear MUM-1 and bcl-6 (Figure 3).
Figure 3.
Dual myeloma-associated antigen-1 (MUM-1)/bcl-6 quantum dot immunofluorescence. Sections were stained with MUM-1 (Qdot 605) and bcl-6 (Qdot655) and counterstained with diamidino-2-phenylindole to highlight nuclei. Images were digitally colorized (MUM-1 green and bcl-6 red). The left panels show MUM-1 alone, middle panel bcl-6 alone, and right panels show combined overlay in the same microscopic field. The top row shows a reactive germinal center from a tonsil in which only rare cells express MUM-1, numerous cells express bcl-6, and only a single cell coexpresses the two proteins (circle). The middle row, from a MUM-1-negative follicular lymphoma (FL), shows malignant FL cells expressing only bcl-6. The bottom row, from a MUM-1-positive FL, shows several MUM-1-expressing cells and numerous bcl-6-expressing cells. The MUM-1 and bcl-6 colocalize (circles).
discussion
Evidence from several recent studies indicates that the OS for patients with FL has improved in recent years [5–7]. This may reflect improvements in treatment, including the introduction of anti-CD20 monoclonal antibody-based therapy. Randomized studies in the first-line and salvage setting have confirmed that the addition of rituximab to standard combination chemotherapy for patients with FL improves event-free survival and OS [2–4].
The FLIPI describes clinical risk factors that are useful in risk stratification for clinical trials in FL and for planning initial therapy for patients with this disease [1]. Although this model has prognostic value in FL, there is marked variability in outcome for patients within each FLIPI risk group, indicating the underlying biological heterogeneity of this disease. The identification of ‘biological’ prognostic factors is therefore essential in FL to allow risk stratification in clinical trials and to identify potential new therapeutic targets in this disease.
Several recent studies have described characteristics of the tumor microenvironment related to host immune response, which may have prognostic value in patients with FL. GEP has identified immune response signatures with prognostic significance which appear to be independent of clinical prognostic factors [8]. The signatures described were not derived from tumor cells but from infiltrating immunoregulatory cells. Two signatures were identified—one composed of genes mainly expressed by T cells and associated with a favorable prognosis and one composed of genes mainly expressed by macrophages and follicular dendritic cells that are associated with an unfavorable prognosis.
Several subsequent studies have used immunohistochemical techniques to investigate the potential prognostic significance of infiltrating cells of T-cell and macrophage lineage in FL. Results from these studies have been variable. In most studies, total T-cell numbers have not been shown to have any prognostic value in FL. By contrast, some studies have reported that the number or pattern of infiltrating FOXP3-positive Tregs is predictive of survival independent of clinical factors [10, 12, 13].
Farinha et al. [9] investigated LAM content in 61 patients treated between 1987 and 1993 with a multi-agent chemotherapy regimen followed by involved-field radiation therapy. In this study, the presence of <15 CD68-positive macrophages per hpf was associated with a favorable prognosis. This retained significance in multivariate analysis which included IPI score.
More recent studies have indicated that the prognostic significance of the tumor microenvironment in FL may be treatment dependent. de Jong et al. [16] have reported that patterns of infiltration with FOXP3-positive T cells and CD68-positive macrophages have opposite prognostic significance in patients treated with alkylating agent-based therapy compared with purine analogue-based therapy. Canioni et al. [15] have demonstrated that high macrophage counts were associated with poor prognosis in patients with FL treated with chemotherapy alone but that this effect was apparently abrogated in patients receiving rituximab in addition to chemotherapy. Median follow-up in this study was relatively short at 42.9 months.
In contrast to most of the previously reported studies, the current series includes patients treated in a consistent, protocol-directed fashion, before and after the addition of monoclonal antibody therapy, in the context of prospective clinical trials with long-term follow-up. We found no correlation between number or pattern of FOXP3-positive Tregs with OS. Likewise, the number of LAMs was not associated with OS.
Expression of MUM-1 has been reported in around 15%–40% of cases of FL in several studies [26–29]. Natkunam et al. [27] described an analysis of MUM-1/IRF4 protein expression using TMAs in a range of hematopoietic neoplasms and reported MUM-1 staining in 23% of all FL cases examined, with 79% of MUM-1-positive cases being grade 3 FL. Naresh [29] reported similar findings in 46 patients with FL. Seventeen cases expressed MUM-1, with 76% of MUM-1-positive cases classified as grade 3. The clinical significance of MUM expression has not, to our knowledge, been explored in a uniformly treated group of patients. A single-center study in 94 patients with FL managed with variable strategies identified MUM-1 in 23 of 87 assessable patients (27%) and showed that the presence of MUM-1, although not independently predictive of OS, was associated with a requirement for treatment at the time of diagnosis.
The present study confirms the adverse prognostic impact of MUM-1 positivity in FL. This effect appears to be restricted to patients treated with anti-CD20 monoclonal antibody therapy. The biological basis for this is unclear. Previous studies have indicated that cytologic grade may be increased in MUM-1-positive FL. However, we did not observe this in the present study. A total of only 22 of 168 assessable patients had grade 3 FL (all but one grade 3a) and no correlation was observed between FL grade and either MUM-1 expression or outcome (data not shown).
MUM-1 has been identified as a marker of nongerminal center-derived DLBCL, a subtype also associated with more aggressive clinical behavior and poor prognosis. We demonstrated that MUM-1 and bcl-6 are indeed abnormally coexpressed in the same cells. The basis for abnormal coexpression of MUM-1 and bcl-6 is a matter of current investigation. In the setting of DLBCL, one mechanism appears to be mutation of the MUM-1-binding site within the bcl-6 promoter [30, 31]. Whether this plays a role in FL is yet to be determined. However, preliminary sequencing data from our laboratory indicate that this is not the case in three samples of MUM-1-positive FL since only germline sequence was identified (data not shown).
In summary, we have shown that, in contrast to previous studies, the number or patterns of infiltration FOXP3 cells and LAMs did not have prognostic significance for patients treated on sequential SWOG studies for FL before and after the introduction of anti-CD20 monoclonal antibody therapy. The presence of MUM-1 positivity was shown to be an adverse prognostic factor in FL patients who received monoclonal antibody but not in those treated with chemotherapy alone. The mechanism underlying this is unclear and will require further study. In view of the relatively small patient numbers included in this study, these results should be considered exploratory and will require independent confirmation. If these results are confirmed in future studies, expression of MUM-1 may allow identification of a poor-risk patient population with FL in which novel treatment approaches should be investigated.
funding
National Cancer Institute (PHS Cooperative Agreement grant numbers DHHS CA32102, CA38926, CA114748, CA04919, CA20319, CA11083, and CA13612); Coulter Pharmaceutical, Inc.
Acknowledgments
This work was presented in part at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 2007 and the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 2008.
References
- 1.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 2.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 3.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 4.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 5.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas MD Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 8.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. New Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 9.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 10.Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOX-P3 regulatory T-cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 11.Farinha P, Campo E, Banham A, et al. The architectural pattern of FOXP3+ T-cells is an independent predictor of survival in patients with follicular lymphoma. Mod Pathol. 2006;19:224A. [Google Scholar]
- 12.Lee AM, Clear AJ, Calaminici M, et al. Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol. 2006;24:5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 13.Alvaro T, Lejeune M, Salvado MT, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–5357. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 14.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, et al. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–5789. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 15.Canioni D, Salles G, Mounier N, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 16.de Jong D, Koster A, Hagenbeek A, et al. Impact of tumor microenvironment on prognosis in follicular lymphoma is dependent on specific treatment protocols. Hematologica. 2009;94:70–77. doi: 10.3324/haematol.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley T, Beck R, Absi A, et al. Biologic predictors in follicular lymphoma: importance of markers of immune response. Leuk Lymph. 2007;48:2403–2411. doi: 10.1080/10428190701665954. [DOI] [PubMed] [Google Scholar]
- 18.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mounier N, Briere J, Gisselbreacht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2 associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 20.Fisher RI, Dana BW, LeBlanc M, et al. Interferon alfa consolidation after intensive chemotherapy does not prolong the progression free survival of patients with low-grade non-Hodgkin's lymphoma: results of the Southwest Oncology Group phase III study 8809. J Clin Oncol. 2000;18:2010–2016. doi: 10.1200/JCO.2000.18.10.2010. [DOI] [PubMed] [Google Scholar]
- 21.Maloney DG, Press OW, Braziel RM, et al. A phase II trial of CHOP followed by rituximab chimeric monoclonal anti-CD20 antibody for treatment of newly diagnosed follicular non-Hodgkin's lymphoma: SWOG 9800. Blood. 2001;98:843a. [Google Scholar]
- 22.Press OW, Unger J, Braziel RM, et al. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group protocol S9911. Blood. 2003;102:1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 24.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. New Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 25.Perea G, Altes A, Montoto S, et al. Prognostic indexes in follicular lymphoma: a comparison of different prognostic systems. Ann Oncol. 2005;16:1508–1513. doi: 10.1093/annonc/mdi269. [DOI] [PubMed] [Google Scholar]
- 26.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–2092. [PubMed] [Google Scholar]
- 27.Natkunam Y, Warnke RA, Montgomery K, et al. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod Pathol. 2001;14:686–694. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- 28.Karube K, Guo Y, Suzumiya J, et al. CD10−MUM1+ follicular lymphoma lacks BCL2 gene translocation and shows characteristic biologic and clinical features. Blood. 2007;109:3076–3079. doi: 10.1182/blood-2006-09-045989. [DOI] [PubMed] [Google Scholar]
- 29.Naresh KN. MUM1 expression dichotomizes follicular lymphoma into predominantly, MUM1-negative low grade and MUM-positive high-grade subtypes. Haematologica. 2007;92:267–268. doi: 10.3324/haematol.10682. [DOI] [PubMed] [Google Scholar]
- 30.Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Lossos IS. The endless complexity of lymphocyte differentiation and lymphomagenesis: IRF downregulates BCL6 expression. Cancer Cell. 2007;12:189–191. doi: 10.1016/j.ccr.2007.08.012. [DOI] [PubMed] [Google Scholar]