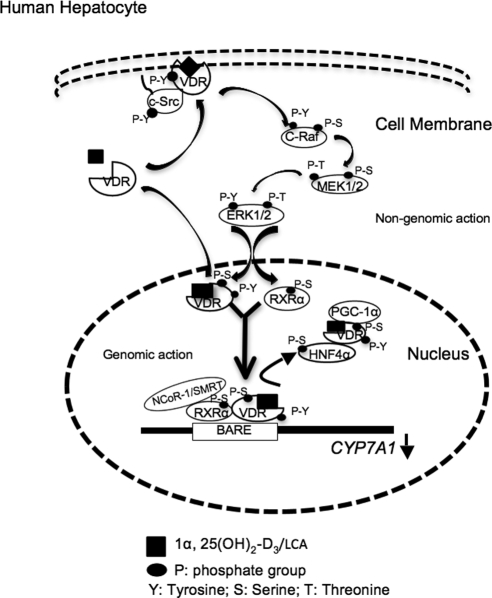
Figure 10.
A cartoon illustrates genomic and nongenomic actions of VDR signaling in inhibition of human CYP7A1 gene transcription in human hepatocytes. VDR ligands, 1α,25(OH)2-D3, or LCA stimulate intracellular translocation of VDR to both the plasma membranes. VDR ligands induce tyrosine (Y) phosphorylation of VDR and activate c-Src. VDR interacts with c-Src in the plasma membrane. Membrane VDR signaling activates c-Raf, probably through activation of the Shc/Grb2/Sos complex and Ras (data not shown), which then activates c-Raf and subsequently the MEK1/2/ERK1/2 MAPK pathway. VDR is also translocated to the nucleus through interaction with a heat shock protein 70, independent of the tyrosine kinase pathway. ERK1/2 is translocated to the nucleus and subsequently phosphorylates the serine/threonine (S/T) residues on nuclear VDR, RXRα, and HNF4α. The VDR/RXRα recruits corepressor NCoR-1 or SMRT to the BARE to displace the HNF4α/VDR/PGC-1α or SRC-1 complex and results in inactivating the CYP7A1 gene. It should be noted that NCoR-1/SMRT is recruited to RXRα that dimerizes with ligand-activated VDR. VDR interacts with HNF4α and prevents HNF4α from dimerization and binding to the BARE.