Abstract
Background and Aims
Using two parental clones of outcrossing Trifolium ambiguum as a potential model system, we examined how during seed development the maternal parent, number of seeds per pod, seed position within the pod, and pod position within the inflorescence influenced individual seed fresh weight, dry weight, water content, germinability, desiccation tolerance, hardseededness, and subsequent longevity of individual seeds.
Methods
Near simultaneous, manual reciprocal crosses were carried out between clonal lines for two experiments. Infructescences were harvested at intervals during seed development. Each individual seed was weighed and then used to determine dry weight or one of the physiological behaviour traits.
Key Results
Whilst population mass maturity was reached at 33–36 days after pollination (DAP), seed-to-seed variation in maximum seed dry weight, when it was achieved, and when maturation drying commenced, was considerable. Individual seeds acquired germinability between 14 and 44 DAP, desiccation tolerance between 30 and 40 DAP, and the capability to become hardseeded between 30 and 47 DAP. The time for viability to fall to 50 % (p50) at 60 % relative humidity and 45 °C increased between 36 and 56 DAP, when the seed coats of most individuals had become dark orange, but declined thereafter. Individual seed f. wt at harvest did not correlate with air-dry storage survival period. Analysing survival data for cohorts of seeds reduced the standard deviation of the normal distribution of seed deaths in time, but no sub-population showed complete uniformity of survival period.
Conclusions
Variation in individual seed behaviours within a developing population is inherent and inevitable. In this outbreeder, there is significant variation in seed longevity which appears dependent on embryo genotype with little effect of maternal genotype or architectural factors.
Keywords: Seed development, seed-to-seed variation, seed longevity, seed coat colour, cohort and population measurements, model system, Trifolium ambiguum
INTRODUCTION
Individual, apparently mature seeds harvested at the same time from a single interbreeding population of mother plants vary considerably in a wide range of traits including, for example, mass (McGinley et al., 1990; Castellanos et al., 2008; Jaradat and Rinke, 2008), level of dormancy/timing of germination (Biere, 1991; Hoyle et al., 2008), and enzyme activity (Still and Bradford, 1997; Mo and Bewley, 2003). Seed-to-seed variation is also evident during seed development. This is surmised from seed development studies where the population mean for traits such as seed mass are usually determined using replicate samples (typically 3–5) of multiple seeds (e.g. Rasyad et al., 1990; Hay and Probert, 1995; Mai-Hong et al., 2003). The standard error of the mean of these measures indicates that seed-to-seed variation occurs even though measurements are not made on individual seeds. This variation reflects differences in the growing environment of the mother plants, the timing of fertilization, and the external and internal environments of the developing seeds. In crop seed-lots the mother plants will have shared a relatively uniform seed production environment, resulting in substantially uniform flowering and fertilization. In non-domesticated species this will be less so. In both, there may be significant effects of seed position within the fruit, fruit position within the inflorescence and position of the inflorescence within the plant – as well as relative timing of fertilization – on seed traits. These effects suggest significant competition for maternal resources between individual seeds.
The breeding system of the maternal plants will also exert significant effects. Seeds are genetic chimeras: the embryo is the diploid product of meiotic segregation and recombination; the endosperm is a triploid tissue resulting from the fusion of two copies of the maternal and one copy of the paternal gamete genomes; and the seed coat is diploid maternal tissue. Repeated high levels of inbreeding, as in the production of crop varieties, will result in seed collections that are essentially genetically homogeneous between and within individuals. Outbreeding, the usual situation in most wild species, will result in the opposite, with considerable levels of genetic heterogeneity between and within each individual in the collection.
In the case of seeds destined for long-term storage, the most significant trait showing seed-to-seed variation, in both crop and wild plant species, is longevity (survival period). The primary tenet of the viability equations (Ellis and Roberts, 1980), widely used to quantify seed longevity, is that the period to seed death is normally distributed within a population of seeds. Probit analysis determines the parameters of this distribution in accordance with the viability equation
| 1 |
where v is the viability [normal equivalent deviates (NED)] of a population of seeds with initial viability Ki (NED) after p days in air-dry storage and σ is the standard deviation of the distribution of seed deaths in time (days) (Ellis and Roberts, 1980). A further tenet of the viability equation is that σ is constant for all seed-lots within a species when placed in an identical storage environment. If seeds are stored in a favourable environment (low temperature, low moisture), the distribution is broadened; σ increases. In a less favourable storage environment, σ decreases. If we could eliminate interseed variation, σ would become zero; all the seeds would die at the same time during storage, regardless of the storage environment. The length of the lag before this catastrophic viability loss would be the parameter that changed depending on the storage environment. Furthermore, a seed-lot would ideally be harvested when all the seeds have attained the same maximum potential longevity.
Seed development studies can attempt either to limit sources of interseed variation or to study their effects explicitly. Most studies of seed developmental physiology have employed crop cultivars as models. In consequence, studies have tended to be skewed towards seeds where the seed coat and the embryo genotypes are identical and near homozygous. To better understand the interplay between maternal and zygotic genotypes which can be expected in the vast majority of non-domesticated species, which mainly outbreed, we have undertaken seed development and seed quality studies at the level of single seeds on Trifolium ambiguum (Caucasian or Kura clover; Bryant, 1974; Taylor and Smith, 1998; Abberton et al., 1998), a highly outbreeding species, which we have used as a model system. To achieve this, we have grown and near-simultaneously reciprocally crossed two cloned lines under conditions which allowed other potential sources of variation to be controlled or quantified.
We chose T. ambiguum as our study species for the following reasons. (a) Within the Fabaceae the genus Trifolium lies in the same sub-clade as Pisum, Medicago and Vicia, while Phaseolus, Glycine and Vigna lie only two clades away. Hence behaviours similar to cultivated grain legumes can be expected. (b) It is self-infertile and so, if bees are excluded, hand pollination will ensure that variation in pollination time is minimized. For pollinations carried out at the same time, the environmental conditions during subsequent seed development will be identical. (c) Large numbers of genetically identical parent plants can be clonally propagated. (d) Each parent plant produces many inflorescences each with many flowers: sufficient flowers are available for experimental purposes within the restricted pollination period. (e) The ovary of each flower has only one or two ovules: any variation due to sequential fertilization can be easily observed. (f) The seeds are non-endospermic with the endosperm substantially absorbed early in development and remaining only as an aleurone layer in the mature seed. (g) The dormancy and germination characteristics of seeds of Fabaceae species are well understood.
We report this model system and the variation in seed characters during seed development. The parameters measured reflect the behaviours of the different genomes within the seed: seed coat colour and hardseededness reflect the maternal genome; seed weight reflects the interaction between the maternal and filial genomes; and germination, desiccation tolerance, and survival reflect the filial genome. We also studied the factors controlling variation in individual seed life spans (in subsequent experimental air-dry storage) during seed development. Our overall intention is to better understand how to obtain seed-lots of greater uniformity and optimum quality, when collecting seeds for ex situ conservation, particularly of wild outbreeding plant species. As seed coat colour is often used as an indicator of seed maturity, understanding how coat colour changes in relation to quality measures may help to guide collectors in the field, and this was also studied.
MATERIALS AND METHODS
Plant propagation and selection of clones
Approximately 100 scarified seeds of T. ambiguum provided by the Institute of Grassland and Environmental Research (IGER), Aberystwyth (population Ah1471, a diploid variety from Australia) were sown in trays of Medium Grade Compost (William Sinclair Ltd, Lincoln, UK) at a depth of approx. 50 mm on 11 December 2003. The seed trays were placed in a glasshouse at Reading University at 20 °C with continuous supplementary lighting to promote germination. After 35 d, seedlings were transferred to conditions of approx. 15/7 °C (12 h light/12 h dark). Once seedlings had produced two or three trifoliate leaves, individual seedlings were potted up into separate 70 mm diameter pots of Medium Grade Compost (William Sinclair Ltd). A solution of Rhizobium mixture obtained from IGER was watered onto seedlings 42 and 59 d after sowing. Root nodules were evident once plant roots had become established. On 31 March 2004, 30 vigorous plants were selected and transferred to a glasshouse at Wakehurst Place where they were grown under conditions of approx. 20/10 °C (12 h light/12 h dark) with supplementary lighting. Plants were watered as necessary and given weekly liquid feeds of Vitafeed 111 (Vitax Ltd, Coalville, UK) a balanced fertilizer plus trace elements (diluted 1 : 100 in water).
A few of the plants showing the most vigorous growth were selected for clonal multiplication, either by detaching plantlets or by taking cuttings. Rooting powder Rhizopon B 0·2 % (Fargo Ltd, Littlehampton, UK) was applied to the excised end of cuttings. Plantlets and cuttings were potted up into 70 mm diameter pots in a 2 : 1 mixture of Sylvamix potting compost (Melcourt Industries Ltd, Tetbury, UK) and perlite granules (Scotts Miracle-Gro Company, Godalming, UK). Once plants were sufficiently large they were either multiplied again or potted on into 125 mm diameter (1 L) and 140 mm diameter (2 L) pots in a 1 : 23·33 mixture of Osmocot slow release fertilizer (Scotts Miracle-Gro Company, Godalming, UK) and nursery stock compost (Melcourt Industries Ltd, Tetbury, UK). Early in this multiplication process, a couple of plants for each clone were allowed to establish and produce flowers. Reciprocal crosses were then carried out by hand (see below) between clones. Two of the clones that were found to be compatible and produce viable seeds from these reciprocal crosses were selected for further multiplication. Once sufficient plants of each of these two clones were in the largest pots and sufficiently large, flowering was induced by increasing day length to 16 h. Plants were given weekly liquid feeds of Peters Excel (Scotts Miracle-Gro Company, Godalming, UK), diluted 1 : 100 in water. In total eight of the propagated plants of clone 1 (referred to as M1) and ten plants of clone 2 (M2) were used in these experiments.
For expt 2, plants from earlier investigations were cut back and allowed to ‘rest’ at approx. 20/10 °C (12 h light/12 h dark) for at least 2 months. Plants were then re-potted and flowering induced by again increasing day length to 16 h.
Hand pollination
To prevent pollination by bees, or other insects, and enable control of pollination (timing and source of pollen) plants were placed within a cage constructed from stainless steel and 4 × 4 mm mesh netting. The mesh size was sufficient to exclude all insects large enough to pollinate a papillionaceous flower. Manual cross-pollination of the two clones M1 and M2 was carried out on 8 December 2004 (expt 1) or 11 January 2006 (expt 2) following IGER protocols (Michaelson-Yeates, pers. comm.). First, the keel petal of each floret on the recipient (maternal) inflorescence was pushed down with a pair of forceps. The tip of a folded triangular piece of card was then inserted between the standard and keel petals of a few florets on a donor inflorescence in order to pick up pollen which was then deposited on the stigmas of recipient florets. Apart from old florets at the base of the recipient inflorescence and immature florets at the top which were removed using forceps, all the florets on an inflorescence were pollinated. A maximum of five inflorescences were pollinated on each plant. Flowers not pollinated were removed, as were all inflorescences appearing subsequently, thereby reducing competition for assimilates.
Seed harvest
Experiment 1
One inflorescence was taken from one of the M1 plants on each of 14, 22, 28, 30, 33, 36, 40, 44, 47, 50, 54, 58, 61, and 64 days after pollination (DAP). In addition one inflorescence was taken from one of the M2 plants on each of 22, 28, 36, 40, 47, 50, 58, and 61 DAP (fewer M2 inflorescences were available at pollination). For harvests when one inflorescence from both clones were sampled, all the seeds from one inflorescence were processed before harvesting the second. The stems were placed in a beaker of water whilst pods were removed and the seeds dissected out under a light microscope. For each seed, pod position within the inflorescence (top, middle, or bottom), number of seeds within the pod, position within the pod (apical or basal; for single-seeded pods this was not always possible to discern and so was not recorded), and fresh weight (f. wt; to the nearest μg on a seven-place balance; Mettler Toledo, Leicester, UK) were recorded. After weighing, individual seeds were randomly assigned for testing moisture content [and hence dry weight (d. wt)], germination (of fresh seed), or desiccation tolerance (germination after desiccation).
Experiment 2
Seed harvests were made at regular intervals between 20 and 70 DAP. For each harvest, a total of 5–7 inflorescences were removed from the two maternal types (seeds processed as above). Each seed was treated separately, recording the position of the pod in the inflorescence (top, middle, or bottom), number of seeds in the pod (one or two) and their position (apical or basal; only recorded for double-seeded pods), before measuring the seed f. wt to the nearest μg using a seven-place balance (Mettler Toledo). The colour of the testa for each seed was assessed by eye and recorded as green, yellow, dark yellow, orange or dark orange. After weighing, seeds were randomly assigned for testing moisture content (and hence d. wt) and, for seeds harvested on or after 36 DAP, germination of fresh seed, desiccation tolerance, or germination after a variable period of experimental storage. Relatively few dark yellow seeds were harvested overall. Hence, this colour category was not represented in the samples used for moisture content determination.
Dry weight/moisture content determination
Seeds destined for moisture content determination were dried in an oven at 103 °C for 17 h according to the low-constant-temperature-oven method (International Seed Testing Association, 2005). After drying, individual seed d. wt was measured on the seven-place balance as above, and moisture content calculated as a proportion of f. wt.
Germination and desiccation tolerance
Each individual seed was tested for ability to germinate on 1 % dH2O agar held within a single 10 mm diameter well of a multidish culture plate (125 × 85 × 20 mm, height × width × depth; Nunc AIS, Denmark) at 15 °C with light provided for 8 h d−1. Seeds failing to imbibe within 28 d were recorded as hardseeded, then scarified by removing a small portion of the seed coat using a scalpel and placed on fresh agar for a further 28 d.
To test for desiccation tolerance, individual seeds were dried for 28 d in separate empty multidish wells without lids at 20 °C over silica gel in a 170 × 115 × 60 mm (height × width × depth) polyethylene sandwich box sealed with Nescofilm (Bando Chemical Industries Ltd, Hyogo, Japan). They were then tested for germination (and hardseededness) as above.
Seed longevity
Individual seeds that had been dried as above in expt 2 were scarified and returned to their allotted multidish well. The multidish plates were placed, without their lids, at 20 °C over a non-saturated LiCl solution at 47 % relative humidity (RH) (Hay et al., 2008) held within a sealed 300 × 300 × 130 mm electrical enclosure box (Ensto UK Ltd, Southampton, UK). After 20 d equilibration of the seeds, the multidish plates were placed, again uncovered, at 45 °C over a different non-saturated LiCl solution at 60 % RH held in an electrical enclosure box as above. The experimental storage period was deemed to commence on transfer to 45 °C, 60 % RH, the initial treatment at 20 °C, 47 % RH having adjusted seed moisture content to that in equilibrium with 45 °C, 60 % RH (Hay et al., 2008). Random samples of approx. 20 seeds were removed at 3–7 d intervals and tested for germination as above.
Data analysis
All analyses were carried out using GenStat for Windows 11th Edition (VSN International Ltd, Hemel Hempstead, UK). For expt 1, a two-sample Poisson test (normal approximation) was used to compare the numbers of seeds from M1 or M2 inflorescences. χ2 tests were used to compare proportions, e.g. of pods bearing one or two seeds. For both experiments, correlation coefficients were determined by plotting individual seed d. wt against their f. wt within each harvest. The Shapiro–Wilk test was used to test for normality of the distribution of f. wt, d. wt, weight of water, and moisture content within each harvest. The amount of skewness and kurtosis when fitting the normal distribution was also assessed. Where appropriate, i.e. a normal distribution could be fitted, t-tests were used to compare mean seed f. wt or d. wt for sub-sets of data, and analysis of variance (ANOVA; unbalanced design) with harvest age (DAP), maternal parent (M1 or M2), number of seeds per pod, or position in the inflorescence (top, middle, or bottom) was used to identify sources of variation in mean seed f. wt or d. wt. Experimental storage data (expt 2) were subjected to probit analysis in order to fit eqn (1) and estimate p50 [period of experimental storage (in days) for population viability to be reduced to 50 %, the product of Ki and σ]; analysis of deviance with an approximate F-test was used to assess whether or not survival curves could be constrained to a common slope (σ−1).
RESULTS
Trifolium ambiguum as a model system
Trifolium ambiguum was well suited to clonal propagation and hand (and thus controlled) pollination. The mean number of seeds produced by M1 inflorescences (i.e. M1 = maternal parent, pollinated by M2) was 41 in expt 1 and 44 in expt 2 (Supplementary Data Tables S1–3, available online). In experiment 1, M2 inflorescences (i.e. M2 = maternal parent, pollinated by M1) produced significantly fewer seeds (mean of 26; Poisson test; P < 0·001), but this difference was not detected in expt 2 (mean of 48). Two seeds developed in a greater proportion of pods than had been anticipated: in expt 1, 49 and 70 % for M1 and M2 inflorescences, respectively. This maternal parent effect was significant (χ2 = 16·68, 1 d.f., P < 0·001) and also observed in experiment 2. Twice as many seeds were harvested from the middle of the inflorescences than from either above or below (Supplementary Data Tables S1–3). This region had twice the number of florets of each of the other regions available for pollination on the selected date, the florets developing sequentially from bottom to top.
Seed development
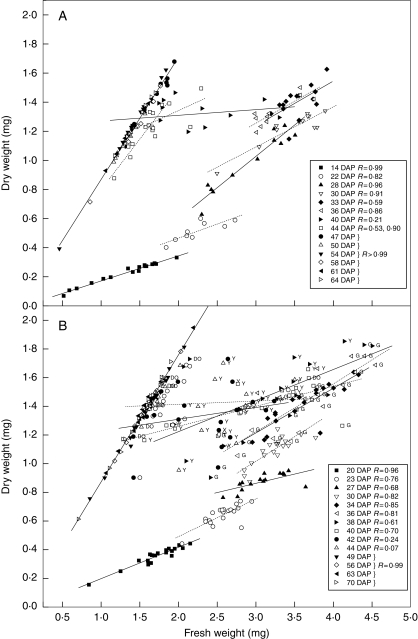
The pattern of increase in seed f. wt and d. wt over time was very similar for the two experiments (Fig. 1). Harvest f. wt and d. wt of individual seeds increased up to 33–36 DAP (also shown in Fig. 2A, F for expt 1). Up to this point, there was good correlation between d. wt and f. wt within each harvest (Fig. 1); however, the relationship changed over time, as seed d. wt and the seed-to-seed variation in d. wt increased. For example, in expt 1, at 14 DAP individual seed d. wt ranged from 0·07 to 0·33 mg; at 28 DAP the range was 0·63–1·24 mg (Fig. 1A).
Fig. 1.
The relationship between individual seed d. wt and f. wt at harvest during seed development in Trifolium ambiguum. (A) Experiment 1, hand pollinated 8 December 2004, harvests from M1 maternal line only. A similar pattern was apparent for seeds with maternal line M2; however, not all harvest dates were represented and hence data are not shown. (B) Experiment 2, hand pollinated 11 January 2006. Correlation coefficients (R values) are within each harvest except for seeds harvested at 44 DAP (expt 1), where a split-line model was used, and for seeds harvested at or after 47 DAP (expt 1) or 49 DAP (expt 2), where the data were combined for the different harvests. In (B), symbol labels for 36–44 DAP indicate seed coat colour as assessed by eye: G, green; Y, yellow; O, orange; DO, dark orange. All seeds at 49–70 DAP were orange or dark orange.
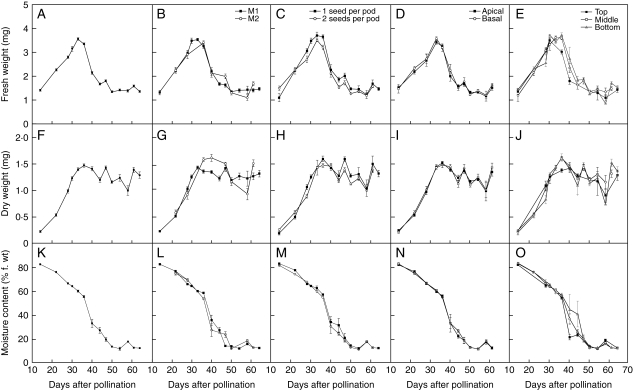
Fig. 2.
Changes in (A–E) harvest f. wt (mean ± s.e.), (F–J) d. wt (mean ±s.e.), and (K–O) moisture content (mean ± s.e.) during seed development in Trifolium ambiguum (expt 1; hand pollinated 8 December 2004). Means calculated for all seeds (A, F, K) or for cohorts sorted by maternal parent (B, G, L), number of seeds per pod (C, H, M), position within the pod (D, I, N), and position within the inflorescence (E, J, O).
Although absolute seed water content rose, relative seed moisture content measured at the population level declined steadily over this seed filling period (Figs 2K and 3). Absolute seed water content then declined, resulting in a more rapid drop in relative seed moisture content until equilibrium (approx. 11 % f. wt) with ambient conditions was reached at 47 DAP (expt 1) or 49 DAP (expt 2). During this phase of water loss, the correlation between f. wt and d. wt within each harvest was compromised. In expt 1, the lowest R value was 0·21 at 40 DAP (Fig. 1A) and the standard error for the mean moisture content was greater at 40 DAP than any other harvest date (Fig. 2K). After 47 or 49 DAP (expts 1 and 2, respectively), there was once again good correlation between individual seed d. wt and f. wt (R > 0·99 within each harvest; Fig. 1). There were no further significant changes in either, or, therefore, in moisture content, between these and the respective last harvests (Figs 2A, F, K and 3).
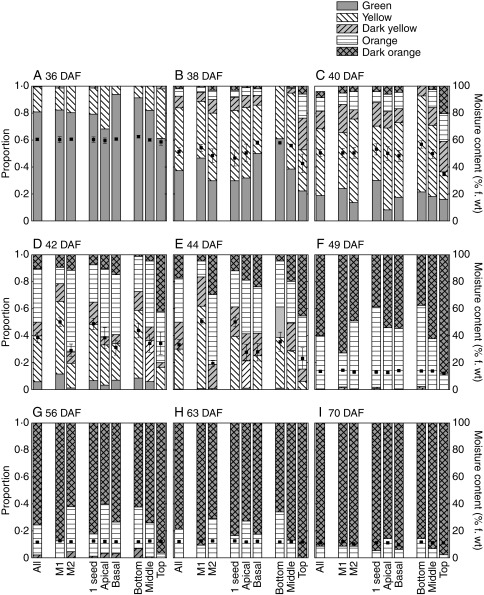
Fig. 3.
The proportions of Trifolium ambiguum seeds from expt 2 (pollinated 11 January 2006) falling into five colour categories (bars, left-hand axis) and the mean seed moisture content (±s.e.; squares, right hand axis). Proportions shown for all seeds (first column) and for cohorts sorted depending on whether they were harvested from M1 or M2 maternal parents; from a single-seeded pod or position within the pod if two seeded; and from the bottom, middle, or top of the inflorescence. Each moisture content shown was determined with approx. 20 seeds; no seeds taken from the top of the inflorescence at 63 DAF were used for moisture content determination.
Individual seed f. wt and d. wt within each harvest could generally be fitted using normal distributions (Table 1). However, in the phase after population mass maturity when mean seed moisture content was falling rapidly (40–47 or 49 DAP, for expts 1 or 2, respectively), there were high levels of skewness and/or kurtosis for individual seed water content (actual mass and/or as a proportion of f. wt). Harvest time accounted for most of the variation in f. wt (86 and 68 % in expts 1 and 2, respectively), but there were also significant main effects of maternal parent (P < 0·05; expt 1 only), number of seeds per pod (P < 0·01), and position in the inflorescence (P < 0·001; Table 2). For example, seeds from the top of the inflorescence tended to have lower f. wt than those from the middle or bottom (Fig. 2E). There were also significant interactions between harvest time and maternal parent (P < 0·05; Table 2) or position in the inflorescence (P < 0·001; expt 1 only). Harvest time was also the largest source of variation in mean seed d. wt (37 %) between 33 and 64 DAP in expt 1 (Table 2), appearing to fluctuate between sequential harvests (Fig. 2F), but not in expt 2 (as expected, since by 36 DAP mass maturity had been reached). Maternal parent and number of seeds per pod were significant factors (P < 0·001) in both experiments (Table 2). Differences in mean seed moisture contents within each harvest depending on maternal parent, number of seeds per pod, and position within the pod were small or nil (shown for expt 1 only; Fig. 2L–N). However, the rate of decline in moisture content after 36 DAP appeared to vary depending on the position within the inflorescence; desiccation occurred progressively more rapidly from the top to the bottom of the inflorescence (Fig. 2O).
Table 1.
Results of the Sharpiro-Wilk test for normality (W) and the skewness and kurtosis of fitted normal distributions for the four developmental parameters indicated
| Fresh weight (mg) |
Dry weight (mg) |
Mass of water (mg) |
Moisture content (% f. wt) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAP | W | Skew. | Kurt. | W | Skew. | Kurt. | W | Skew. | Kurt. | W | Skew. | Kurt. |
| Experiment 1 (pollinated 8 December 2004) | ||||||||||||
| 14 | 0·9018 | −0·65 | −0·83 | 0·8910* | −0·82 | −0·56 | 0·9038 | −0·61 | −0·88 | 0·8171** | 1·80 | 4·15 |
| 22 | 0·9658 | −0·18 | −1·12 | 0·9412 | −0·34 | −1·23 | 0·9412 | 0·38 | −0·60 | 0·9622 | −0·18 | −1·11 |
| 28 | 0·8988* | −0·50 | −1·15 | 0·9527 | −0·47 | −0·85 | 0·9368 | −0·33 | −1·15 | 0·9623 | 0·37 | −0·37 |
| 30 | 0·8457* | −1·27 | 0·67 | 0·7862** | −1·65 | 1·83 | 0·8923 | −0·89 | −0·15 | 0·9258 | 0·58 | −0·46 |
| 33 | 0·9590 | 0·21 | −1·15 | 0·9533 | −0·32 | 0·75 | 0·9162 | −0·01 | −1·56 | 0·8112** | 1·20 | 0·16 |
| 36 | 0·9401 | −0·59 | 0·03 | 0·9589 | −0·17 | −1·16 | 0·9444 | −0·91 | 0·75 | 0·9318 | −0·91 | 1·06 |
| 40 | 0·9434 | 0·62 | −0·42 | 0·9623 | 0·50 | −0·29 | 0·8698* | 1·08 | 0·13 | 0·8828* | 0·49 | −1·34 |
| 44 | 0·9429 | 0·49 | 0·17 | 0·9345 | −0·82 | 0·32 | 0·7855*** | 1·42 | 0·71 | 0·7636*** | 1·92 | 3·16 |
| 47 | 0·9198 | 0·61 | −0·15 | 0·9159* | −0·84 | −0·21 | 0·7398*** | 1·46 | 0·72 | 0·7630*** | 1·40 | 0·62 |
| 50 | 0·9402 | 0·22 | −1·14 | 0·9555 | 0·19 | −1·07 | 0·9488 | 0·50 | −0·84 | 0·9582 | 0·07 | −1·03 |
| 54 | 0·7926 | −1·70 | 4·07 | 0·7922** | −1·72 | 4·16 | 0·8613* | −1·05 | 2·72 | 0·8656* | 1·06 | 0·06 |
| 58 | 0·9092 | −0·64 | −0·89 | 0·9184 | −0·60 | −0·92 | 0·8880 | −0·87 | −0·52 | 0·8415** | 0·88 | −0·65 |
| 61 | 0·9664 | 0·07 | −0·56 | 0·9639 | 0·14 | −0·59 | 0·9791 | −0·20 | 0·21 | 0·9693 | −0·24 | −0·91 |
| 64 | 0·9460 | −0·34 | −1·42 | 0·9297 | −0·33 | −1·51 | 0·9915 | −0·27 | −0·96 | 0·9723 | −0·18 | −1·42 |
| Experiment 2 (pollinated 11 January 2006) | ||||||||||||
| 20 | 0·8886* | −1·32 | 1·86 | 0·8782* | −1·39 | 1·82 | 0·8996* | −1·24 | 1·63 | 0·9190 | 0·48 | −0·91 |
| 23 | 0·9691 | −0·47 | 0·14 | 0·9141 | −1·01 | 0·97 | 0·9875 | 0·17 | −0·35 | 0·8325** | 1·55 | 1·91 |
| 27 | 0·9679 | 0·16 | −0·99 | 0·9262 | −0·53 | −0·80 | 0·9561 | 0·58 | −0·31 | 0·8267** | 1·57 | 2·94 |
| 30 | 0·8382** | −1·64 | 3·83 | 0·8158** | −1·79 | 3·19 | 0·8825* | −1·02 | 3·01 | 0·9691 | 0·18 | −0·13 |
| 34 | 0·9565 | 0·13 | −1·21 | 0·9472 | −0·28 | −1·11 | 0·9250 | 0·31 | −1·32 | 0·8566* | 1·48 | 2·97 |
| 36 | 0·9758 | −0·06 | −1·00 | 0·9506 | −0·26 | −1·10 | 0·9703 | −0·30 | −0·80 | 0·9515 | −0·80 | 0·62 |
| 38 | 0·9552 | 0·01 | −1·11 | 0·9518 | −0·43 | −0·85 | 0·9418 | −0·56 | −0·44 | 0·7138*** | −1·76 | 1·84 |
| 40 | 0·9407 | −0·40 | −1·03 | 0·9553 | 0·01 | −1·29 | 0·9601 | −0·19 | −0·68 | 0·8810* | −0·85 | −0·08 |
| 42 | 0·9343 | −0·09 | −1·32 | 0·9757 | −0·09 | −1·32 | 0·8961* | −0·01 | −1·61 | 0·8857* | −0·24 | −1·53 |
| 44 | 0·9276 | 0·53 | −0·77 | 0·9531 | −0·66 | 0 | 0·8546** | 0·36 | −1·38 | 0·8209** | 0·09 | −1·80 |
| 49 | 0·9222 | −0·96 | 0·85 | 0·9306 | −0·84 | 0·53 | 0·8949* | −1·05 | 1·17 | 0·8130** | 1·90 | 4·71 |
| 56 | 0·9631 | −0·24 | 0·67 | 0·9569 | −0·27 | 0·72 | 0·9538 | 0·19 | −1·06 | 0·9260 | 0·66 | −0·28 |
| 63 | 0·9166 | 0·35 | 1·84 | 0·9150 | 0·45 | 1·97 | 0·9649 | −0·02 | −0·79 | 0·9775 | 0·04 | −0·68 |
| 70 | 0·8922* | −1·23 | 1·32 | 0·9011* | −1·21 | 1·32 | 0·9671 | −0·58 | −0·13 | 0·9702 | 0·05 | −0·88 |
Data shown are for all seeds harvested on a given date (and so M1 only for 14, 30, 33, 44, 54, and 64 DAP in expt 1).
A significant value for the W-statistic indicates non-normality. *P <0·05; **P <0·01; ***P <0·001.
Table 2.
Accumulated analysis of variation (unbalanced design therefore fitted through GenStat regression) for fresh weight and dry weight of seeds of Trifolium ambiguum harvested between 33 and 64 DAP (expt 1) or between 36 and 63 DAP (expt 2).
| Experiment 1 (pollinated 8 December 2004) |
Experiment 2 (pollinated 11 January 2006) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh weight (mg) |
Dry weight (mg) |
Fresh weight (mg) |
Dry weight (mg) |
|||||||||||||
| Change | d.f. | SS | MS | VR | d.f. | SS | MS | VR | d.f. | SS | MS | VR | d.f. | SS | MS | VR |
| + DAP | 9 | 118·80 | 3·20 | 200·46*** | 7 | 3·35 | 0·48 | 24·28*** | 7 | 86·82 | 12·40 | 65·06*** | 7 | 0·26 | 0·04 | 1·34 |
| + Mat. | 1 | 0·34 | 0·34 | 5·13* | 1 | 0·31 | 0·31 | 15·65*** | 1 | 0·06 | 0·06 | 0·29 | 1 | 1·18 | 1·18 | 41·88*** |
| + No. | 1 | 2·23 | 2·23 | 33·81*** | 1 | 0·46 | 0·46 | 23·13*** | 1 | 1·92 | 1·92 | 10·07** | 1 | 0·59 | 0·59 | 21·11*** |
| + PosI | 2 | 1·74 | 0·87 | 13·20*** | 2 | 0·05 | 0·02 | 1·16 | 2 | 6·64 | 3·32 | 17·42*** | 2 | 0·20 | 0·10 | 3·48* |
| + DAP × Mat. | 5 | 0·84 | 0·17 | 2·54* | 4 | 0·68 | 0·17 | 8·63*** | 7 | 5·51 | 0·79 | 4·13*** | 7 | 0·47 | 0·07 | 2·39* |
| + DAP × No. | 8 | 0·93 | 0·12 | 1·76 | 7 | 0·38 | 0·05 | 2·78* | 7 | 1·42 | 0·20 | 1·07 | 7 | 0·26 | 0·04 | 1·32 |
| + Mat. × No. | 1 | 0·06 | 0·06 | 0·98 | 1 | 0·00 | 0·00 | 0·18 | 1 | 0·01 | 0·01 | 0·08 | 1 | 0·05 | 0·05 | 1·95 |
| + DAP × PosI | 14 | 3·48 | 0·25 | 3·77*** | 12 | 0·82 | 0·07 | 3·48*** | 13 | 3·68 | 0·28 | 1·48 | 13 | 0·42 | 0·03 | 1·16 |
| + Mat. × PosI | 2 | 0·25 | 0·13 | 1·93 | 2 | 0·01 | 0·00 | 0·16 | 2 | 0·63 | 0·32 | 1·66 | 2 | 0·11 | 0·06 | 1·98 |
| + No. × PosI | 2 | 0·10 | 0·05 | 0·73 | 2 | 0·14 | 0·07 | 3·61* | 2 | 0·20 | 0·10 | 0·53 | 2 | 0·02 | 0·01 | 0·37 |
| + DAP × Mat. × No. | 3 | 0·05 | 0·02 | 0·25 | 2 | 0·04 | 0·02 | 1·04 | 6 | 0·57 | 0·09 | 0·49 | 6 | 0·36 | 0·06 | 2·12 |
| + DAP × Mat. × PosI | 3 | 0·30 | 0·10 | 1·53 | 2 | 0·06 | 0·03 | 1·44 | 7 | 1·65 | 0·24 | 1·24 | 7 | 0·12 | 0·02 | 0·60 |
| + DAP × No. × PosI | 3 | 1·01 | 0·34 | 5·14** | 2 | 0·47 | 0·24 | 11·92*** | 7 | 0·62 | 0·09 | 0·46 | 7 | 0·13 | 0·02 | 0·67 |
| + Mat. × No. × PosI | 1 | 0·31 | 0·31 | 4·78* | 1 | 0·18 | 0·18 | 9·36** | 1 | 1·15 | 1·15 | 6·03 | 1 | 0·00 | 0·00 | 0·13 |
| + DAP × Mat. × No. × PosI | 1 | 0·02 | 0·02 | 0·35 | 0 | 0·00 | 0·00 | 0 | 0 | 0 | 0 | 0 | ||||
| Residual | 128 | 8·43 | 0·07 | 105 | 2·07 | 0·02 | 92 | 17·54 | 0·19 | 92 | 2·59 | 0·03 | ||||
| Total | 184 | 138·89 | 0·75 | 151 | 9·03 | 0·06 | 156 | 128·42 | 0·82 | 156 | 6·77 | 0·04 | ||||
DAP, days after pollination; Mat., maternity (M1 or M2); No., seed number; PosI, position in inflorescence (top, middle, or bottom).
*P < 0·05; **P < 0·01; ***P < 0·001.
Position in the pod was not always recorded for single-seeded pods and therefore this factor was not included in the analysis. Changing the order of factors did alter the significance of some second-order interaction terms, but not of main effects or first-order interactions.
Seed coat colour (expt 2 only)
The relative proportions of seeds with different coat colours through the harvesting period reflected the sequence of colour change (green → yellow → dark yellow → orange → dark orange; Figs 1B and 3). At 36 DAP most of the seeds were green and a small proportion yellow; at 44 DAP, half the seeds were yellow or dark yellow, and the remainder were orange or dark orange. By 56 DAP most of the seeds were dark orange; however, a uniform population of dark orange seeds was never achieved, even at the last harvest (70 DAP). Categorizing seeds according to colour meant that they were also, largely, sorted into groups that reflected their moisture content (Table 3; also apparent in Fig. 1B where individual seeds that were more advanced in the colour series tended to have the lower f. wt within their harvest cohort). Green seeds harvested at between 36 and 42 DAP consistently had the highest moisture content (mean within each harvest >60 %; Table 3). The moisture content of yellow seeds tended to be lower than that of green seeds; this difference was significant at 36 DAF (t-test, P < 0·05; the difference was not significant or could not be tested for other harvest dates). Similarly, the moisture content of orange seeds was generally less than that of yellow seeds at each harvest (significantly different at 44 DAP; t-test, P < 0·05). By 44 DAP there was no difference in the moisture contents of orange and dark orange seeds at each harvest.
Table 3.
Moisture content (% f. wt; mean ± s.e.) of Trifolium ambiguum seeds harvested at different stages during development and sorted by seed coat colour (expt 2; hand-pollinated 11 January 2006)
| DAP | Green | Yellow | Orange | Dark orange |
|---|---|---|---|---|
| 36 | 61·5 ± 0·74† | 55·6 ± 2·74a‡ | – | – |
| 38 | 60·2 ± 0·69† | 51·8 ± 1·85† | 18·8‡ | 19·2‡ |
| 40 | 60·4 ± 3·50† | 48·2± 2·84** | 43·0 ± 11·71‡ | 56·6‡ |
| 42 | 61·2‡ | 48·6 ± 2·61* | 24·1 ± 3·73† | 15·4 ± 0·60‡ |
| 44 | – | 47·6 ± 2·47† | 15·9 ± 0·50† | 15·4 ± 0·66† |
| 49 | – | – | 13·8 ± 0·54** | 12·9 ± 0·38† |
| 56 | – | 12·0‡ | 11·9 ± 0·20‡ | 12·5 ± 0·25† |
| 63 | – | – | 11·5 ± 0·47† | 12·1 ± 0·41† |
| 70 | – | – | 12·5 ± 0·42‡ | 11·2 ± 0·35† |
The Shapiro–Wilk test for normality was used for each DAP × colour category: test-statistic; *P <0·05; **P <0·0.; †Non-significant; ‡Test not carried out if <3 seeds within the category; if only one seed, no standard error is shown.
The moisture content declined within each colour category as seeds were harvested progressively later. For example, at 36 DAP yellow seeds had a mean moisture content of 55·6 %, whereas at 44 DAP it was 47·6 % (Table 3). Sub-sets of seeds which showed more rapid decline in moisture content also progressed more quickly through the sequence of colour change. For example, at 42 DAF, the ratio of green : yellow : dark yellow : orange : dark orange seeds from M1 plants (mean moisture content 50·0 %) was 0·12 : 0·54 : 0·13 : 0·12 : 0·09 (i.e. most seeds were yellow; Fig. 3D) compared with 0·01 : 0·18 : 0·08 : 0·61 : 0·12 (most seeds orange) for seeds from M2 plants (mean moisture content 29·2 %). These differences in colour progression and moisture content between seeds from M1 and M2 plants were also apparent at 38 and 44 DAF; for subsequent harvests there were no differences in mean seed moisture content between seeds from M1 and M2 plants and, if anything, seeds from M2 plants appeared less mature, based on their colour (Fig. 3).
The colour ratios also suggested that seeds from two-seeded pods matured more quickly than those from single-seeded pods between 40 and 49 DAF (Fig. 3D–F). There was not a consistent trend in the proportions of seeds in each colour category depending on whether they came from the apical or basal position of the pod (seeds from two-seeded pods only; Fig. 3). Seeds from the top of the inflorescence appeared to mature more quickly than seeds from the bottom or middle of the inflorescence throughout the developmental period studied. This was also reflected in lower moisture contents for seeds from the top of the inflorescence.
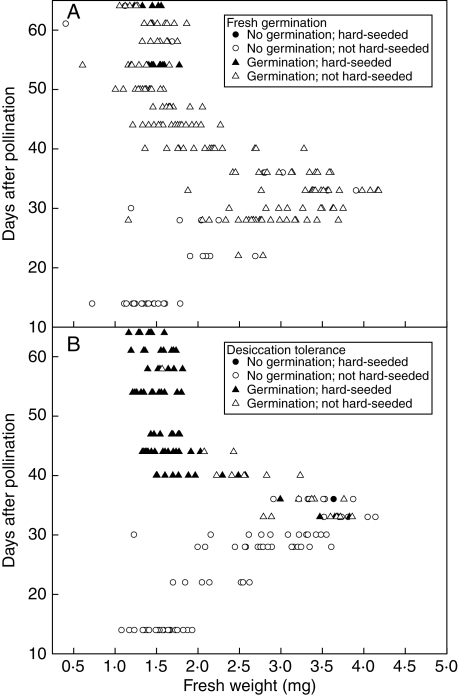
Germinability, desiccation tolerance and hardseededness
In expt 1, ability to germinate was first detected at 22 DAP; most seeds were able to germinate by 28 DAP but the ability of all seeds to germinate was not observed until 42 DAP (Figs 4A and 5A). The ability to tolerate desiccation was first seen in some individuals at 33 DAP, with all individuals desiccation tolerant 7 d later (Figs 4F and 5B). Thus some seeds appear to acquire desiccation tolerance 11 d after germinability whilst later maturing seeds acquire germinability and desiccation tolerance almost simultaneously or possibly even in a different order (i.e. desiccation tolerance first). No consistent differences were detected in these patterns between seeds from M1 and M2 inflorescences (Fig. 4B,G). Comparisons amongst individual seeds within harvests between 22 and 30 DAP suggested that it might be the heavier seeds within populations that were capable of germinating (only shown for seeds with maternal line M1 and thus only for 28 and 30 DAP; Fig. 5A). However, at 22 DAP, when ability to germinate was 31 %, the difference in mean f. wt between germinating and non-germinating seeds was not significant (t-test; P > 0·05). At 28 and 30 DAP, the mean f. wt of seeds able to germinate was greater (P < 0·05) than that of those which did not, but in both these cases the numbers of non-germinators was small (Fig. 5A). Similarly, visual examination of the results for individuals within a harvest over the period when populations began to acquire desiccation tolerance (at 33 and 36 DAP for seeds from maternal line M1; Fig. 5B) suggested that this might be acquired earlier in seeds that had begun to dry sooner, but this was not significant (P > 0·05).
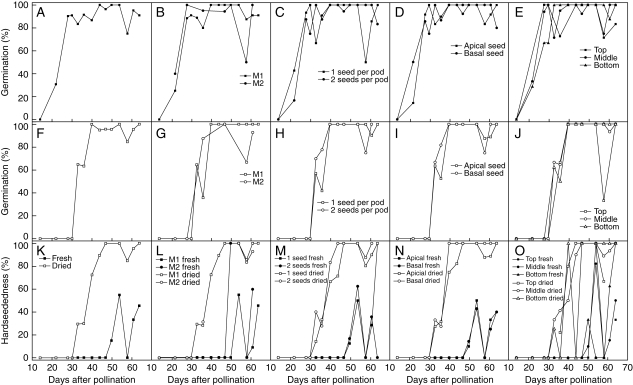
Fig. 4.
Changes in (A–E) fresh seed germination, (F–J) desiccation tolerance (seeds dried over silica gel at 20 °C for 28 d), (K–O) hardseededness of fresh (filled symbols) or dried (open symbols) seeds during seed development in Trifolium ambiguum (expt 1, pollinated 8 December 2004). Proportions shown for all seeds (A, F, K) or for cohorts sorted by maternal parent (B, G, L), number of seeds per pod (C, H, M), position within the pod (D, I, N), or position within the inflorescence (E, J, O).
Fig. 5.
The f. wt of individual seeds of Trifolium ambiguum (expt 1, pollinated 8 December 2004) harvested between 14 and 64 DAP from the M1 maternal line that did or did not germinate and were or were not hardseeded when placed to germinate (A) immediately after harvest or (B) after drying over silica gel (for 28 d at 20 °C).
Seeds that developed within double-seeded pods appeared to acquire the ability to germinate slightly later than those from one-seeded pods (Fig. 4C), whereas if anything the former may have tolerated desiccation slightly earlier than those from one-seeded pods (Fig. 4H). The effect of seed position within double-seeded pods had a similarly negligible but also potentially contradictory effect. Ability to germinate may have been acquired slightly earlier across the population by seeds from the base of pods (Fig. 4D), whereas desiccation tolerance may have been acquired slightly sooner in apical seeds (Fig. 4I). Position in the inflorescence gave the greatest effect on the development of ability to germinate: seeds that developed in the top of the inflorescence acquired this some 2 d earlier than those in the middle, whilst those at the bottom were some 2–5 d later than those in the middle (Fig. 4E). In contrast, the development of desiccation tolerance was unaffected by inflorescence position (Fig. 4J). Number of seeds per pod, position within the pod and position within the inflorescence had no effect on the incidence of hardseededness (Fig. 3M–O).
In expt 2, harvesting seeds for germination and experimental storage did not commence until 36 DAP, when 83 % of fresh seeds had acquired germinability and 67 % were already desiccation tolerant (Supplementary Data Table S3, available online). All seeds achieved germinability and desiccation tolerance at 40 and 42 DAP, respectively. In the case of seeds harvested at 36 DAP, there was no difference in the mean seed f. wt of seeds that did germinate compared with those that failed to germinate, either before (mean ± s.e., 3·7 ± 0·11 mg for seeds that germinated compared with 3·6 ± 0·24 mg for those that did not) or after drying (mean f. wt of seeds that were desiccation tolerant 3·6 ± 0·08 mg compared with 3·8 ± 0·09 mg for seeds that were not; Supplementary Data Table S3). Seeds that did not germinate fresh at 36 DAP had green testas (Supplementary Data Table S3). Similarly, the seeds that had not acquired desiccation tolerance at 36 and 38 DAP were green, even though by 38 DAP there were some seeds in all categories of coat colour, with the largest proportion being yellow.
On-plant hardseededness developed during late development and maturation (only recorded for expt 1; Figs 4K and 5A), as individual seed moisture content approached equilibrium with ambient. Comparison of the hardseedness results for in planta desiccation with variation in mean moisture content suggests that at moisture contents close to 12 %, hardseededness may be lost or acquired depending on subtle variation in ambient humidity. Whilst the trait was associated with desiccation in planta, it was expressed earlier in development and in a much greater proportion of the population when dried ex planta (28 d at 20 °C over silica gel; Figs 4K and 5B). The developmental trend for hardseededness when dried ex planta generally followed the development of the ability to tolerate desiccation; in some individuals both traits may be acquired almost simultaneously whilst in others hardseededness is delayed by up to 7 d (Fig. 4K). The development of hardseededness upon drying did not differ between seeds from M1 or M2 maternal parents (Fig. 4L). The apparent difference in hardseededness of fresh seeds harvested at 50 DAP from different maternal lines may not be reliable due to low sample size (all three M2 seeds were hardseeded whilst none of the 17 M1 seeds was hardseeded).
Seed longevity (expt 2 only)
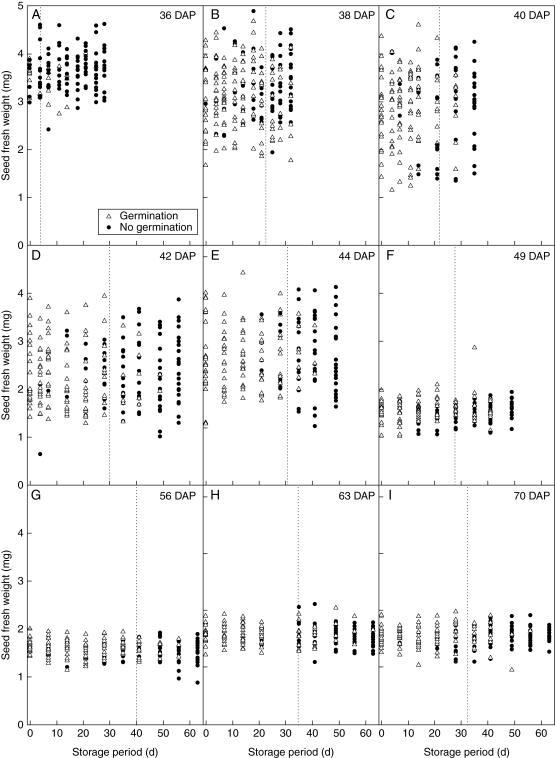
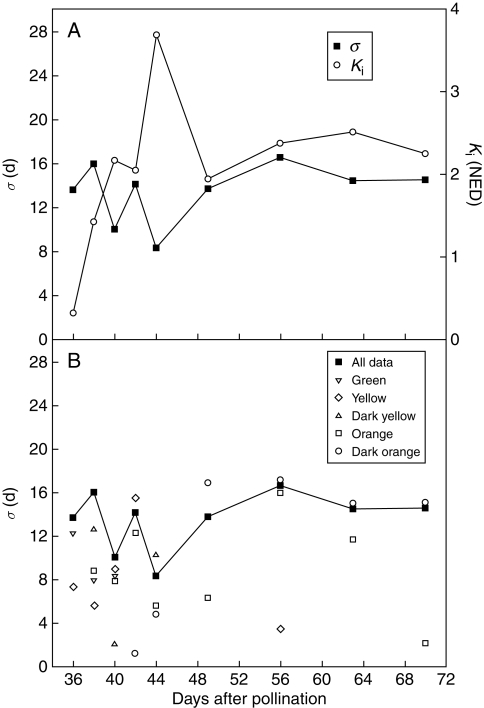
Seed longevity in experimental storage (60 % RH, 45 °C) increased progressively between 36 (seed-lot p50 = 4·3 d) and 56 DAP (seed-lot p50 = 40·1 d), declining progressively thereafter until 70 DAP (seed-lot p50 = 32·5 d) (Fig. 6). The harvest f. wt of seeds that germinated at each sample time during experimental ageing tended to be higher than the f. wt of those that failed to germinate at 36 and 38 DAP (Fig. 6A, B). However, these differences were not significant (t-test, P > 0·05) and were not consistently apparent for later harvests (Fig. 6C–I). The large increase in seed-lot p50 between 36 and 44 DAP was due to an increase in Ki [eqn. (1)], from 0·31 to 3·7 NED (Fig. 7A). It subsequently fell to an apparent plateau of approx. 2·2 NED between 49 and 70 DAP. The estimates for σ fluctuated between 8 and 16 d. However, it was possible to constrain the survival curves for all the harvest times to a common estimate for σ without a significant increase in the residual deviance [P > 0·05; σ−1 = 0·0733 (s.e. 0·00332)], such that all the variation in survival among harvest dates was accounted for by differences in the estimates of Ki.
Fig. 6.
The f. wt of individual seeds of Trifolium ambiguum (expt 2, pollinated 11 January 2006) harvested between 36 and 70 DAP that did (open triangles) or did not (filled circles) germinate after different experimental storage periods (x-axis) at 60 % RH and 45 °C. The vertical dotted lines indicate the time when viability had fallen to 50 % (seed-lot p50), estimated by probit analysis.
Fig. 7.
Changes in longevity parameters during seed development in Trifolium ambiguum (expt 2, pollinated 11 January 2006). (A) Estimates of Ki and σ, as indicated, when all the data within each harvest were included in the probit analysis. (B) Estimates of σ for cohorts of the population sorted according to seed coat colour. The common value of σ was 13·6 d (see text).
For seeds harvested at 36 DAP, a greater proportion of yellow seeds were able to germinate upon removal from experimental storage at each sampling time compared with those that were green (Supplementary Data Table S3). Similarly for 38 DAP seeds, those that were green were less likely to germinate when removed from experimental storage. However, there was little consistent difference in the survival of yellow, dark yellow, orange, or dark orange seeds within each subsequent harvest. The estimate of σ for colour-sorted cohorts of the population fluctuated in a similar way to that when the data were not sorted, but was generally lower, particularly for harvests between 36 and 44 DAP (Fig. 7). This apparently smaller amount of seed-to-seed variation in longevity within colour-sorted cohorts may be due to lower seed numbers within each cohort. Low seed numbers similarly confound the possibility of assessing the extent to which factors such as maternal clone or plant, number of seeds per pod, etc. influence seed longevity. For many of the analyses of the experimental storage data where seeds were allocated to two or more sub-populations, there was not a significant increase in residual deviance if the sub-populations were modelled using a common slope or even a single line through all the data.
DISCUSSION
Seed development
Few studies of physiological seed development have looked at the level of individual seeds. Those that have been reported have been carried out on crop species (e.g. Munier-Jolain and Ney, 1998; Ishimaru et al., 2003). We chose to study T. ambiguum as a model for other outbreeding, genetically heterozygous species, with the aim of increasing our understanding of the genotypic and architectural variables that affect the seed-to-seed variability in physiological traits and, hence, we surmise, influence seed development. This understanding may help to optimize the quality of seed collections.
Working at either a population or individual seed level, it is not possible to monitor the same set of individuals through their development. Seed development studies inevitably require that seeds are removed from the maternal plant and the measurement of many of the parameters is destructive (including germination). Thus, such studies assume that all seeds follow the same development sequence, and time is used as the independent variable. Recognizing that the development of individual seeds may vary, we have considered the use of individual seed f. wt at harvest, a non-destructive measurement, as an alternative internal (i.e. characteristic of the seed) independent variable to chart seed development. Additionally we have monitored changes in seed coat colour to reflect changes in the state of the maternal tissue and to understand the relationship between this maternal tissue and the behaviour of the enclosed embryo.
Using population measurements of developmental parameters, by calculating the means or overall percentages of the measurements made on individual seeds, seed development over time in T. ambiguum is similar to that of other orthodox species (Figs 2A, F, K and 4A, F). A key point in population studies of seed development is the cessation of seed filling and achievement of mass maturity, defined as the attainment of maximum d. wt (Ellis and Pieta Filho, 1992). A single value (±s.e.) is usually given as the maximum d. wt for the population based on samples taken at regular intervals during seed development. Adopting this whole-population approach, our results show that mass maturity occurred between 33 and 36 DAP when seeds reached a mean d. wt of 1·47 mg (shown for expt 1 only; Fig. 2F). Achievement of mass maturity was followed by a rapid decline in seed moisture content (Fig. 2K), ‘maturation drying’ (Kermode and Bewley, 1985). However, it is clear these population parameters can be misleading at the individual seed level. Comparing the range in individual seed d. wt for seeds that were either in the process of drying or had dried to equilibrium with ambient humidity, some seeds can be deduced to have reached their ‘individual’ maximum d. wt as early as 28 DAP in expt 1 (Fig. 1A) or 27 DAP in expt 2 (Fig. 1B). Identifying the latest individuals to achieve their maximum d. wt is problematic. Despite having a near common time of pollination, seeds that were still increasing in d. wt at, for example, 30 DAP and those that had already achieved their relatively low maximum d. wt and had started to dry could not be distinguished with certainty. It is perhaps more likely that those individuals that had the fastest rate of seed filling were also those that were going to reach a higher maximum d. wt and would therefore be the heaviest within any harvest.
In our study we used only two maternal lines. ANOVA showed that at the population level, harvest date, maternal parent, number of seeds per pod, position within the inflorescence, and the interactions of these factors accounted for large proportions of the variation in individual seed harvest f. wt (94 and 86 % for expts 1 and 2, respectively) and d. wt (77 and 62 %) over the developmental periods studied (Table 2). More rigorous partitioning of the phenotypic variance would require more lines and all combinations of reciprocal crossing. Furthermore, the efforts to eliminate any confounding effects of microclimate by randomizing and/or rotating the positioning of maternal parents within the glasshouse would need to be greater than here. Nonetheless our results are consistent with the results of Castellanos et al. (2008), who found strong maternal effects, accounting for 59 % of the phenotypic variation, in dispersal mass (analogous to our d. wt measurements) of seeds of Aquilegia pyrenaica subsp. cazorlensis, but did not rule out zygotic genotype effects. The residual variation we observed suggests that the embryo genotype is to some degree determining final seed mass in T. ambiguum. Looking at individual seeds also showed that, despite the small variation in pollination times, the variation in d. wt increased during seed development (Fig. 1). This was consistent with variation in the rate of filling between individual seeds. The similarity in seed d. wt between one- and two-seeded pods (Fig. 2H), between apical and basal seeds (of two-seeded pods; Fig. 2I), and between seeds taken from different parts of the inflorescence (Fig. 2J) implies little competition for assimilates. Nonetheless, ANOVA indicated that these factors were significant sources of variation. Overall the rate of seed filling appeared to vary between individuals and depended not only on maternal genotype or environment and architectural effects, but also on the embryo genotype. Furthermore, the wide variation in d. wt of individual seeds within each maternal clone suggests an interaction between embryo and maternal genotypes. This is inconsistent with the maternal genotype influencing seed size by controlling embryo cell numbers as reported for genetically homogenous pea cultivars (Lemontey et al., 2000).
The accumulation of seed d. wt ceases when the abscission layer forms within the maternal tissue and cuts off the supply of water and nutrients to the developing seed. From population studies, all seeds are thought to start losing water at this point and eventually equilibrate with ambient humidity (Le Deunff and Rachidian, 1988). Our data (Fig. 1) suggest that the two processes are independent to a degree. Thus, as with variation between individual seeds in the timing of individual seed mass maturity, there is variation in the timing of the onset of maturation drying, despite the maternal genotype being identical. The non-normality of the water content of individual seeds during the period of rapid drying (Table 1) reflects the interactions between the similar maternal tissue and the genetically varied embryos. The rate at which seeds dry may also vary, and again this may be determined by the embryo genotype or due to architectural/microenvironmental effects. Whilst there was little effect of seed number per pod (Fig. 2M) or position within a pod (Fig. 2N), seeds from the top of the inflorescence appeared to dry more quickly than seeds lower down the inflorescence (Fig. 2O). The radiation incident upon inflorescences may have declined progressively from top to bottom. If so, the temperature and relative humidity of the air surrounding pods would have declined and increased, respectively, from the top to the bottom of inflorescences, resulting in the variation in rate of maturation drying observed.
The maturation drying period was also the phase when seed coat colour changes were occurring, but again at different times for different individuals (Fig. 1B) and to some extent depending on maternal parent and position within the inflorescence from which the seed was taken (Fig. 3A–F). Although seed coat colour was not a precise indicator of seed moisture content across harvest times, within each harvest time mean seed moisture content sequentially differed for each colour category, being highest for green seeds and lowest for dark orange seeds (Table 3). Seed coat colour change has been associated with changes in physiological activity and the onset of drying in other legumes. For example, in Glycine max the respiration rate decreased as the seed coat changed from yellow-green to yellow (TeKrony et al., 1979). Hyde et al. (1959) reported that seed coat colour in T. pratense and T. repens started to change when the mean seed d. wt was 80 % of the d. wt at population mass maturity. In T. ambiguum, we might now interpret this variation in seed coat colour to reflect some individuals having reached their individual mass maturity earlier, together with an earlier onset of drying. This again suggests an interaction between the embryo and the maternal seed coat which contrasts with the controlling role of the seed coat reported for seed filling in genetically homogeneous cultivars of legumes evolutionarily close to Trifolium (Lemontey et al., 2000). The acquisition of desiccation tolerance largely occurred just prior to the seed coat turning from green to yellow (Fig. 1), although this was not a definitive marker in all individuals; some green seeds were desiccation tolerant at 36 and 38 DAP.
Germinability was first observed at 22 DAP (expt 1; Figs 4A and 5A), before any individual seeds reached their maximum d. wt; however, it was not necessarily the seeds with greatest harvest f. wt that were more physiologically advanced (Fig. 5B). Rather, it could have been those seeds that were closer to reaching their individual mass maturity that acquired germinability sooner. The onset of germinability occurred at essentially the same time for seeds from M1 and M2 maternal inflorescences, suggesting that maternal genotype is not significant. Differences in architectural factors such as number of seeds per pod or position of the seed within the pod (Fig. 4B–D) also had little effect. However, seeds from the bottom of the inflorescence appeared to acquire germinability 3–5 d later than seeds from the middle or top of the inflorescence (Fig. 4E).
Desiccation tolerance was first observed at 33 DAP (expt 1; Figs 4F and 5B), 11 d after germinability was first observed. Again, those seeds that acquired desiccation tolerance the soonest may have been the individuals that reached their maximum d. wt earlier and started maturation drying earlier; the f. wt of individual 33 and 36 DAP seeds that germinated after rapid enforced drying tended to be towards the lower part of the f. wt range (Fig. 5B). This would again suggest some embryo genotype control. Looking at the population level, Sinniah et al. (1998) found that ending irrigation progressively earlier to plants of Brassica campestris resulted in maturation drying, onset of ability to germinate and onset of desiccation tolerance all occurring sooner. However, whilst T. ambiguum seeds at the top of the inflorescence dried more rapidly (Fig. 2O), there is no evidence that they acquired desiccation tolerance sooner (Fig. 4J).
Hardseededness in planta was aquired late during development, with 55 % of the seeds harvested at 54 DAP failing to imbibe when placed, fresh, on agar (Fig. 4K). However, all seeds harvested at 58 DAP did imbibe; this mirrors the reversible hardseededness reported in Lupinus digitatus (Gladstones, 1958). This loss of hardseededness coincided with an increase in seed moisture content (Fig. 2K), presumably a consequence of high ambient humidity at that time. At 54 DAP, mean seed moisture content reached 12·2 %, similar to the onset moisture content for hardseededness reported in other legumes, e.g. 15 % in Peltophorum pterocarpum (Mai-Hong et al., 2003); 12 % in Pisum sativum (Ellis and Roberts, 1982), Lupinus arboreus, Trifolium pratense and T. repens (Hyde, 1954); and 11 % in L. digitatus (Gladstones, 1958). With enforced desiccation post-harvest, however, hardseededness was induced considerably earlier (from 33 DAP) in some individuals and almost coincident with their acquisition of desiccation tolerance. That is, the ability of embryos to survive considerable enforced, rapid desiccation and of testas to become impermeable in response to extreme desiccation developed at similar points during seed development and maturation. This is compatible with seed development studies in Ornithopus compressus (Revell et al., 1999), Lathyrus maritimus and Lathyrus sativus (Chinnasamy and Bal, 2003). Because of the way hardseededness can be overcome in legumes it is usually considered a characteristic of the testa and hence maternal genotype. Since in our experiments there are only two maternal genotypes, we might expect there to be two sub-populations within each of which hardseededness affects all individuals identically. As this is not the case (Fig. 4L), this suggests the origins of hardseededness might be due to other factors we were unable to consider (such as location of the inflorescence on the plant) and/or under embryonic control, this capability developing between 33 and 47 d (Fig. 5B). Callose has been proposed as the cause of hardseededness in other Trifolium species (Bhalla and Slattery, 1984). Our results suggest that embryos of different genotypes exude callose at different times during these early stages of maturation and the callose is then ‘cured’ by drying to produce the impermeable testa. In planta the variation in the frequency of expression of hardseededness and its reversibility suggests that the curing of the callose in the testa may be dependent on the extent of desiccation, its duration or some combination of them.
Whilst limited as an internal non-destructive parameter of development, f. wt when combined with more traditional measures such as period since pollination and if applied at the single seed level can provide insights into the developmental physiology of seeds (of outbreeding plant species). As Fig. 1A shows, a seed with f. wt of 2·5 mg could have been harvested any time between 22 and 40 DAP (expt 1); however, only those harvested at 40 DAP might have been desiccation tolerant. When the other non-destructive measure of the condition of the maternal tissue, seed coat colour, is also taken into consideration, the diagnosis becomes more certain.
Seed longevity
The longevity of a population of developing seeds increases over time. Using probit analysis to fit survival curves to all the experimental storage data within each harvest, the estimate of time for viability of the seed population to fall to 50 % (p50), increased from 4·3 d at 36 DAP to 40·1 d at 56 DAP (Fig. 3). The improvement in longevity (in subsequent experimental storage) for 20 or so days after population mass maturity in T. ambiguum seeds is also similar to that of other species (e.g. Pieta Filho and Ellis, 1991; Demir and Ellis, 1992a, b; Hay and Probert, 1995). Thus, the optimum time to harvest seeds of T. ambiguum in order to have maximum longevity coincided with the seeds reaching equilibrium with ambient conditions and was after all the seed coats had become orange or dark orange (Figs 1B and 3). In most wild plant species, reaching moisture equilibrium with ambient is also when the seeds will reach the point of natural dispersal (Hay and Smith, 2003). However the T. ambiguum pods did not dehisce and there were on-plant declines in longevity between 56 and 70 DAP; seed-lot p50 dropped to 33 d (Fig. 6). On-plant decline in longevity is characteristic of species, mainly crops, which desiccate considerably but have been selected to not release their seeds. For example, on-plant declines in seed longevity were reported in millet (Pennisetum glaucum; Kameswara Rao et al., 1991) and barley (Hordeum vulgare; Pieta Filho and Ellis, 1991). Between 36 and 44 DAP, increases in seed-lot p50 were largely due to increases in Ki [eqn. (1)], presumably as all the developing seeds finally acquired desiccation tolerance (Fig. 7A). Ki can be a more sensitive measure of the desiccation tolerance of a population of seeds than a germination test that uses a relatively small number of dried seeds, provided that the most advanced seeds have not started to lose viability.
There is not yet any means to identify the exact moment when individual seeds lose the ability to germinate. Further, in the most immature T. ambiguum seeds tested for longevity (36 DAP), it was not possible to distinguish between those seeds that died early in experimental storage and those that had not acquired desiccation tolerance (Fig. 6A). For seeds harvested at 40 DAP, there was evidence that some seeds had lost the ability to germinate by the time that the 7 d sample was withdrawn from experimental storage (Fig. 6C). However, some individuals were still able to germinate after 28 d in storage. Similarly, for seeds harvested at 63 DAP, some seeds lost the ability to germinate after between 14 and 21 d in experimental storage, but one seed was still able to germinate after 63 d (Fig. 6H). Given their common albeit heterozygous parents and their near synchronous fertilization, this 3- to 4-fold variation in individual seed longevity indicates the large effects that genotype and maternal environment can have on this seed behaviour.
This variation in the time to seed death can be assessed by σ [eqn. (1)]. For seeds from 195 geographically and taxonomically diverse species, estimates for σ under the same storage conditions as used here ranged between 0·9 and 397 d (actual σ values not reported, but see Probert et al., 2009). For eight legume species, the predicted value of σ under these experimental storage conditions ranged between 11 (Arachis hypogaea) and 72 (Vigna radiata) days. The estimate for another Trifolium species, T. subterraneum, was 15 d [estimated using the ‘predict days to lose 1 probit’ module of the Seed Information Database (Liu et al., 2008)]. Here, in T. ambiguum, the population estimates for σ ranged between 8·3 and 16·6 d (Fig. 7A). For all seed-lots (i.e. populations of mature seeds) within a species, the value σ is expected to be constant if they are stored under precisely the same storage conditions (Ellis and Roberts, 1980). This was also the case here during seed development: the T. ambiguum survival curves for the different harvest times (not shown) could be constrained to a common estimate of σ (13·6 d) without a significant increase in residual deviance (P >0·05). Comparing the common estimate of σ for T. ambiguum with that for T. subterraneum, we have perhaps slightly reduced seed-to-seed variation in the time to seed death – by synchronizing pollination to an 8 h period and only using two parental lines – but it has certainly not been eliminated. We have not determined σ for T. ambiguum seed-lots produced following normal collecting (and growing) practice.
The f. wt distributions for seeds that did germinate did not differ from those that did not, for each period of experimental storage and within each harvest (Fig. 6). For instance, for seeds harvested at 36 DAP, when some seeds may not have reached their individual mass maturity, the stored seeds that did not germinate and were perhaps not desiccation tolerant did not have the highest (i.e. had not started to dry) harvest f. wt (Fig. 6A). Individual seed f. wt was not a reliable indicator of individual seed storability. Furthermore, for T. ambiguum seeds that had dried to equilibrium with ambient by the time they are harvested (49–70 DAP), greater d. wt did not infer greater life span (Fig. 6). Thus a similar statement can be inferred for f. wt since after 49 DAP, f. wt and d. wt are correlated (Fig. 1B).
Even for highly bred crop species, which are largely genetically homogeneous, a seed-lot where all the seeds lose the ability to germinate at the same time (i.e. a square survival ‘curve’ with a long lag before very rapid decline in germination) has not been reported. However, in outbreeding species, it would seem that, rather than variation in life span being simply a reflection of physiological maturity, not all seeds within a developing population have the potential to acquire the same level of resistance to ageing in dry storage. Individual seed life span may be another trait that, like final seed mass, is determined by the unique genotype of the embryo. Furthermore, longevity is likely to be under multigene control. In rice, three quantitative trait loci for seed longevity, mapped to different chromosomes, have been identified (Miura et al., 2002; Xue et al., 2008). The lack of reduced seed-to-seed variation in life span of seeds of crop species suggests that although genetically homogeneous, the variation of seed maturation environment significantly affects individual seed longevity.
We did attempt to identify whether any of the factors that were a source of variation in individual seed f. wt or d. wt also influenced seed-to-seed variation in life span, by looking at the survival data for cohorts of seeds within each harvest. However, consistent trends across the harvest dates could not be detected (not shown; Butler, 2007). The estimates for σ were almost always lower for sub-populations compared with the estimates for the whole population at each harvest, suggesting that there was greater synchronization in the time to seed death. However, the estimates could usually be constrained to common values for the different cohorts (P >0·05) and the lower estimates for σ are more likely to be due to having fewer data rather than a real reduction in variation in individual seed life spans.
Trifolium ambiguum as a model system
Overall, controlled crossing of clonally propagated self-incompatible T. ambiguum provided an appropriate model system for studying individual seed development. This model species provides a valuable contrast to the restricted view gained from the genetically homogeneous seeds of frequently studied crop species. Already some improvements to our experimental design have become clear. Each harvest involved sampling entire inflorescences and processing each seed individually; the number of seeds included in the experiments was limited by the time required to process individual seeds rather than by the number of seeds available for observation. However, the number of seeds and pods varied between harvests, as did the number of seeds falling into each cohort, and some combinations of maternal parent, number of seeds per pod, position within the inflorescence, and position within the pod were not represented at each harvest in expt 1. A better sampling procedure may have been to sample pods from across the population, ensuring good representation of all the factor combinations, although this may have had an impact on the development of those which remained to be sampled later. Rather more two-seeded pods were produced than expected. This may have been the result of selecting the most vigorous clones and then selecting the pair of clones with the greatest seed set. Nonetheless, the effect of number of seeds per pod on the developmental parameters studied was comparatively small. Perhaps surprisingly (given the potential for competition for assimilates within a pod), this was as true for seed d. wt as it was for, say, the development of desiccation tolerance.
By growing the plants in a controlled environment and synchronizing (to within 8 h) pollination, we aimed to investigate the extent to which seed-to-seed variability could be reduced in the developing population of T. ambiguum seeds. However, significant variation in physical and physiological development remained. Despite using only two parental lines, since T. ambiguum is an outbreeder, the heterozygosity within these parental genotypes created populations of genetically heterogeneous embryos whose genotypes exerted significantly varied effects on seed development. The effects of number of seeds per pod, seed position within the pod, or position of the pod on the inflorescence, or their interactions with harvest time were relatively small, albeit in some cases significant, possibly because later flowers were removed, so ensuring that resources to the developing seeds were not limited. The extent to which the picture presented here would change in a more natural, open-pollinated situation, where more competition and greater main or interaction effects of these architectural factors could be anticipated, remains unclear.
Conclusions
Whilst not without some difficulties of interpretation, we have shown that a tightly defined seed production system combined with both destructive and non-destructive measurements allows the physiology of seed development to be studied at the level of individual seeds. We have shown the following.
(a) The behaviour of individual seeds varied greatly compared with that which might be expected by extrapolation from the population means.
(b) The different physiological stages of seed development can be significantly varied temporally between individuals.
(c) Development processes appeared to be under the control of both genetic and maternal environment factors. There is little evidence that these maternal factors are due to limited supplies of resource, rather they appear more associated with maternal plant architecture.
(d) Interaction between the maternal and filial genomes during development can be deduced to account for the variation in the completion of seed filling and the commencement of maturation drying between individual seeds.
(e) From our results we deduce that the variation found in individual seed behaviours within a population of outbreeding species could be expected in the variation between seed populations of varieties of inbreeding crop species provided they have not been unintentionally lost during the processes of domestication and breeding.
(f) As we concluded elsewhere (Butler et al., 2009) and has been demonstrated by others (Still and Bradford, 1997; Mo and Bewley, 2003), care should be taken when drawing conclusions from studies using samples of multiple seeds and calculating mean values or proportions.
(g) Individuals within a population of developing seeds differ with respect to the potential life span that they can acquire, and that variation is likely to be influenced by the embryo genotype.
(h) The value of σ for T. ambiguum seeds aged at 60 % RH and 45 °C did not appear to be reduced by controlling the time of pollination and using only two maternal lines. Since it may not be possible ever to make a collection of seeds with uniform life span, those involved in the storage of seed can continue to exploit the normally distributed variation and use the time when viability of an accession falls to 85 % germination to indicate the point when the percentage viability of a seed-lot starts to decline rapidly and thus a prompt for regeneration or recollection of that accession.
Our results also show the value of understanding the genetic background of the different seed tissues when interpreting physiological behaviour. Both inbreeding homogeneous crops and highly outbreeding undomesticated species produce seed development plots of similar shape which require different interpretation. In our highly controlled experiments with simultaneously pollinated T. ambiguum flowers, explanations of the variation in behaviour between individual seeds can properly include variation in embryo genotype as well as factors of plant architecture and internal maternal environment. At the other end of the spectrum, in self-pollinating crops, similar seed-to-seed variations will need to be exclusively interpreted in terms of the timing of fertilization and the external and internal maternal environments. The possibility that these variations can also vary between seasons and locations makes the likelihood of repeatedly creating seed-lots of similar population structure small. This frustrates attempts to understand the molecular behaviour of seeds through the homogenization of many individuals from a population, in a way similar to how the identification of mass maturity in the population obscures the variation in individual maturities.
SUPPLEMENTARY DATA
Supplementary Data are available online at www.aob.oxfordjournals.org and consist of the following tables. Table S1. ‘Passport information’ including harvest fresh weight, dry weight, and moisture content of individual Trifolium ambiguum seeds harvested between 14 and 64 DAP (expt 1, pollinated 8 December 2004). Table S2. Harvest fresh weight, dry weight, and moisture content of individual Trifolium ambiguum seeds harvested between 20 and 70 DAP (expt 2, pollinated 11 January 2006). Table S3. ‘Passport data’ recorded for each individual Trifolium ambiguum seed harvested between 36 and 70 DAP (expt 2, pollinated 11 January 2006) and tested for fresh or dry germination or germination after the period of dry storage indicated [‘time aged’ (at 60 % RH, 45 °C)].
Supplementary Material
ACKNOWLEDGEMENTS
We thank Terry Michaelson-Yeates and his colleagues at IGER for providing advice and the original seeds of Trifolium ambiguum; Jo Walmisley for tending the clover plants; colleagues and students in the Seed Conservation Department who helped with hand pollination; and Rosemary Newton for editing the figures. This work was supported by a research studentship to L.H.B. from the University of Reading Research Endowment Trust and from the Seed Conservation Department, RBG Kew. The Seed Conservation Department receives financial support from the Millennium Commission, The Wellcome Trust and Orange plc., and through grant-in-aid from the Department for Environment, Food and Rural Affairs, UK.
LITERATURE CITED
- Abberton MT, Michaelson-Yeates TPT, Marshall AH, Holdbrook-Smith K, Rhodes I. Morphological characteristics of hybrids between white clover, Trifolium repens L. and Caucasian clover, Trifolium ambiguum M. Bieb. Plant Breeding. 1998;117:494–496. [Google Scholar]
- Biere A. Parental effects in Lychnis flos-cuculi. I: Seed size, germination and seedling performance in a controlled environment. Journal of Evolutionary Biology. 1991;3:447–465. [Google Scholar]
- Bhalla PL, Slattery HD. Callose deposits make clover seeds impermeable to water. Annals of Botany. 1984;53:125–128. [Google Scholar]
- Bryant WG. Caucasian clover (Trifolium ambiguum Beib.) – a review. Journal of the Australian Institute of Agricultural Science. 1974;40:11–19. [Google Scholar]
- Butler LH. Seed development, rehydration and variation in seed survival. 2007 PhD Thesis, University of Reading, UK. [Google Scholar]
- Butler LH, Hay FR, Ellis RH, Smith RD, Murray TB. Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Annals of Botany. 2009;103:1261–1270. doi: 10.1093/aob/mcp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos MC, Medrano M, Herrera CM. Subindividual variation and genetic versus environmental effects on seed traits in a European Aquilegia. Botany. 2008;86:1125–1132. [Google Scholar]
- Chinnasamy G, Bal AK. The pattern of seed development and maturation in beach pea (Lathyrus maritimus) Canadian Journal of Botany. 2003;81:531–540. [Google Scholar]
- Demir I, Ellis RH. Development of pepper (Capsicum annuum) seed quality. Annals of Applied Biology. 1992a;121:385–399. [Google Scholar]
- Demir I, Ellis RH. Changes in seed quality during seed development and maturation in tomato. Seed Science Research. 1992b;2:81–87. [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- Ellis RH, Roberts EH. Desiccation, rehydration, germination, imbibition injury and longevity of pea seeds (Pisum sativum) Seed Science and Technology. 1982;10:501–508. [Google Scholar]
- Ellis RH, Pieta Filho C. Seed development and cereal seed longevity. Seed Science Research. 1992;2:9–15. [Google Scholar]
- Gladstones JS. The influence of temperature and humidity in storage on seed viability and hard-seededness in the west Australian blue lupin, Lupinus digitatus Forsk. Australian Journal of Agricultural Research. 1958;9:171–181. [Google Scholar]
- Hay FR, Probert RJ. The effect of different drying conditions and maturity on desiccation tolerance and seed longevity in Digitalis purpurea L. Annals of Botany. 1995;76:639–647. [Google Scholar]
- Hay FR, Smith RD. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew, UK: Royal Botanic Gardens; 2003. pp. 97–133. [Google Scholar]
- Hay F, Adams J, Manger K, Probert R. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology. 2008;36:737–746. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. Pre- and post-harvest influences on physiological dormancy alleviation of an Australian Asteraceaea species: Actinobole uliginosum (A. Gray) H. Eichler. Seed Science Research. 2008;18:191–199. [Google Scholar]
- Hyde EOC. The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Annals of Botany. 1954;18:241–256. [Google Scholar]
- Hyde EOC, Allison McLeavey M, Harris GS. Seed development in ryegrass and in red and white clover. New Zealand Journal of Agricultural Research. 1959;2:947–952. [Google Scholar]
- International Seed Testing Association (ISTA) International rules for seed testing. Edition 2005. Bassesdorf, Switzerland: The International Seed Testing Association; 2005. [Google Scholar]
- Ishimaru T, Matsuda T, Ohsugi R, Yamagishi T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Functional Plant Biology. 2003;30:1139–1149. doi: 10.1071/FP03122. [DOI] [PubMed] [Google Scholar]
- Jaradat AA, Rinke JL. Flowering, capsule and seed characteristics in Cuphea. Euphytica. 2008;161:447–459. [Google Scholar]
- Kameswara Rao N, Appa Rao S, Mengesha MH, Ellis RH. Longevity of pearl millet (Pennisetum glaucum R.Br.) seeds harvested at different stages of maturity. Annals of Applied Biology. 1991;119:97–103. [Google Scholar]
- Kermode AR, Bewley JD. The role of maturation drying in the transition from seed development to germination. Journal of Experimental Botany. 1985;36:1906–1915. [Google Scholar]
- LeDeunff YL, Rachidian Z. Interruption of water delivery at physiological maturity is essential for seed development, germination and seedling growth in pea (Pisum sativum L.) Journal of Experimental Botany. 1988;39:1221–1230. [Google Scholar]
- Lemontey C, Mousset-Declas C, Munier-Jolain N, Boutin JP. Maternal genotype influences pea seed size by controlling both mitotic activity during early embryogenesis and final endoreduplication level/cotyledon cell size in mature seed. Journal of Experimental Botany. 2000;51:167–175. doi: 10.1093/jexbot/51.343.167. [DOI] [PubMed] [Google Scholar]
- Liu K, Eastwood RJ, Flynn S, Turner RM, Stuppy WH. Seed Information Database. 2008 (release 7.1). http://www.kew.org/data/sid . [Google Scholar]
- Mai-Hong T, Hong TD, Hien NT, Ellis RH. Onset of germinability, desiccation tolerance and hardseededness in developing seeds of Peltophorum pterocarpum (DC) K. Heyne (Caesalpinioideae) Seed Science Research. 2003;13:323–327. [Google Scholar]
- McGinley MA, Smith CC, Elliott PF, Higgins JJ. Morphological constraints on seed mass in lodgepole pine. Functional Ecology. 1990;4:183–192. [Google Scholar]
- Miura K, Lin SY, Yano M, Nagamine T. Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.) Theoretical and Applied Genetics. 2002;104:981–986. doi: 10.1007/s00122-002-0872-x. [DOI] [PubMed] [Google Scholar]
- Mo B, Bewley JD. The relationship between β-mannosidase and endo-β-mannanase activities in tomato seeds during and following germination: a comparison of seed populations and individual seeds. Journal of Experimental Botany. 2003;54:2503–2510. doi: 10.1093/jxb/erg274. [DOI] [PubMed] [Google Scholar]
- Munier-Jolain NG, Ney B. Seed growth rate in grain legumes II. Seed growth rate depends on cotyledon cell number. Journal of Experimental Botany. 1998;49:1971–1976. [Google Scholar]
- Pieta Filho C, Ellis RH. The development of seed quality in spring barley in four environments. I. Germination and longevity. Seed Science Research. 1991;1:163–177. [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasyad A, VanSanford A, TeKrony DM. Changes in seed viability and vigour during wheat seed maturation. Seed Science and Technology. 1990;18:259–267. [Google Scholar]
- Revell CK, Taylor GB, Cocks PS. Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research. 1999;50:1211–1223. [Google Scholar]
- Sinniah UR, Ellis RH, John P. Irrigation and seed quality development in rapid-cycling Brassica: seed germination and longevity. Annals of Botany. 1998;82:309–314. [Google Scholar]
- Still DW, Bradford KJ. Endo-β-mannanase activity from individual tomato endosperm caps and radicle tips in relation to germination rates. Plant Physiology. 1997;113:21–29. doi: 10.1104/pp.113.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NL, Smith RR. Kura clover (Trifolium ambiguum M.B.) breeding, culture, and utilization. Advances in Agronomy. 1998;63:153–178. [Google Scholar]
- TeKrony DM, Egli DB, Balles J, Pfeiffer T, Fellows RJ. Physiological maturity in soybean. Agronomy Journal. 1979;71:771–775. [Google Scholar]
- Xue Y, Zhang SQ, Yao QH, et al. Identification of quantitative trait loci for seed storability in rice (Oryza sativa L.) Euphytica. 2008;164:739–744. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.