Abstract
While most cigarette smokers endorse a desire to quit smoking, only about 14% to 49% will achieve abstinence after 6 months or more of treatment. A greater understanding of the effects of smoking on brain function may (in conjunction with other lines of research) result in improved pharmacological (and behavioral) interventions. Many research groups have examined the effects of acute and chronic nicotine/cigarette exposure on brain activity using functional imaging; the purpose of this paper is to synthesize findings from such studies and present a coherent model of brain function in smokers. Responses to acute administration of nicotine/smoking include: a reduction in global brain activity; activation of the prefrontal cortex, thalamus, and visual system; activation of the thalamus and visual cortex during visual cognitive tasks; and increased dopamine (DA) concentration in the ventral striatum/nucleus accumbens. Responses to chronic nicotine/cigarette exposure include decreased monoamine oxidase (MAO) A and B activity in the basal ganglia and a reduction in α4β2 nicotinic acetylcholine receptor (nAChR) availability in the thalamus and putamen. Taken together, these findings indicate that smoking enhances neurotransmission through cortico-basal ganglia-thalamic circuits either by direct stimulation of nAChRs, indirect stimulation via DA release or MAO inhibition, or a combination of these factors. Activation of this circuitry may be responsible for the effects of smoking seen in tobacco dependent subjects, such as improvements in attentional performance, mood, anxiety, and irritability.
Keywords: Tobacco dependence, Functional magnetic resonance imaging, Positron emission tomography, Autoradiography, Prefrontal cortex, Review
1. Introduction
Approximately 23% of Americans smoke cigarettes (Balluz et al., 2004). While most smokers endorse a desire to quit (Fiore et al., 2000), very few will actually quit smoking without treatment, and only about 14–49% will achieve abstinence after 6 months or more of effective treatment (Holmes et al., 2004; Hughes et al., 1999; Hurt et al., 1997; Jorenby et al., 1999; Killen et al., 1999, 2000). Because cigarette smoking carries both considerable health risks (Bartal, 2001; Mokdad et al., 2004) and high societal costs (Leistikow et al., 2000a,b), there is an urgent need for improved treatments for this condition. Functional brain imaging (in conjunction with other lines of research) holds great promise for elucidating both brain circuits and molecular targets that mediate the acute effects of cigarette smoking and chronic effects of tobacco dependence. A greater understanding of brain function associated with smoking may result in improved pharmacological (and behavioral) interventions.
Many functional brain imaging studies of tobacco use and dependence have been performed, using four primary imaging modalities: (1) functional magnetic resonance imaging (fMRI), (2) positron emission tomography (PET), (3) single photon emission computed tomography (SPECT), and (4) autoradiography. These imaging modalities have been used to determine relationships between brain function and effects of acute and chronic cigarette smoking and of smoking-related behaviors. For this review paper, the MEDLINE database was searched using keywords for the four imaging techniques mentioned above cross-referenced with the words “nicotine,” “cigarette,” and “tobacco.” Only data driven functional imaging studies are included in this review, and reference lists within papers found on MEDLINE were also examined and relevant studies included here. In order to maintain focus in this review paper, functional imaging techniques that provide measures of blood flow and metabolism (which are closely related under normal conditions (Paulson, 2002)) are combined under the general heading of brain activity (including fMRI and certain types of SPECT, PET, and autoradiography studies). Also, in order to build a cohesive model of brain activity responses to acute and chronic smoking, nicotine and cigarette studies will be reviewed together, while recognizing that cigarette smoke has many constituents other than nicotine (Baker et al., 2004; Fowles and Dybing, 2003).
The purpose of this paper is to synthesize findings from functional brain imaging studies of tobacco use and dependence, and present a coherent model of brain function in smokers. Acute brain responses to nicotine/smoking will be reviewed first, followed by chronic responses to nicotine/smoking, and concluding with a discussion of these imaging findings in the context of neuroanatomical work and the clinical effects of smoking in tobacco dependent subjects.
2. Brain function responses to acute nicotine administration and cigarette smoking
2.1. Brain activity responses to nicotine/cigarette administration
Many functional brain imaging studies have been performed examining the effects of administration of nicotine or cigarette smoking compared with a placebo or control state (Table 1). Though a wide range of brain regions have been reported to have altered activity in response to nicotine or cigarette smoking, several global and regional findings have been replicated, leading to general conclusions about the acute effects of nicotine or smoking on brain activity.
Table 1.
Functional brain imaging studies of nicotine or cigarette administration
| Authors | Subjects | Method | Intervention | Results |
|---|---|---|---|---|
| London et al (London et al., 1988b; London et al., 1988a) | Rats | 2-deoxy-d-[1-14C]glucose autoradiography |
SC nic (0.1 to 1.75 mg/kg) | ↑nicotine rich regions, including thal, cereb, visual system, others |
| Rourke et al (Rourke et al., 1997) | 8 smokers; 8 former smokers; 17 non-smokers |
iodine-123 iodoamphetamine (IMP) SPECT |
Smokers smoked the morning of the scan; other groups did not |
↓cortical uptake of IMP (a measure of blood flow) in current smokers compared to other groups |
| Stein et al (Stein et al., 1998) | 16 smokers | fMRI | IV nic (0.75- 2.25 mg/70 kg wt) vs. placebo |
↑R NAc and bilateral amyg, cingulate, frontal lobes, thal, others |
| Marenco et al (Marenco et al., 2000) | Rats- chronically nic exposed vs. nic naive |
2-deoxy-d-[1-14C] glucose autoradiography |
SC nic (0.4 mg/kg) vs. saline | ↑thal, superior colliculus in chronically exposed; ↑thal, superior colliculus, medial habenula and dorsal lateral geniculate in nic naive |
| Domino et al (Domino et al., 2000a) | 18 smokers | 15O-PET | Nic nasal spray vs. pepper spray | ↑thal, pons, visual cortex, cereb |
| Domino et al (Domino et al., 2000b) | 11 smokers | FDG-PET | Nic nasal spray vs. pepper spray | Small ↓global; ↑ L IFG, L PC, R thal, visual cortex; ↓-normalized L ins and R inf occ ctx |
| Zubieta et al (Zubieta et al., 2001) | 18 smokers | 15O-PET | Nic nasal spray vs. pepper spray | ↑ anterior thal; ↓-L ant temp and R amyg |
| Rose et al (Rose et al., 2003) | 34 smokers | 15O-PET | Cigarette vs. no nic control conditions |
↑ L frontal factor (incl prefrontal and ACC), ↓-L amyg rCBF |
| Yamamoto et al (Yamamoto et al., 2003) | 10 smokers | 99mTc-ECD SPECT | Cigarette vs. abstinence | ↓global blood flow |
| Stapleton et al (Stapleton et al., 2003a) | 4 smokers; 2 non-smokers | 2 FDG-PETs (fully quantified) | IV nic (1.5 mg) versus placebo | ↓global and most regions studied |
All regional changes represent normalized activity, unless otherwise stated. SC = subcutaneous; nic = nicotine; thal = thalamus; cereb = cerebellum; SPECT = single photon emission computed tomography; fMRI = functional magnetic resonance imaging; IV = intravenous; R = right; L = left; NAc = nucleus accumbens; amyg = amygdala; FDG = 18F-fluorodeoxyglucose; PET = positron emission tomography; IFG = inferior frontal gyrus; PC = posterior cingulate; ins = insula; inf occ ctx = inferior occipital cortex; ant = anterior; temp = temporal lobe; ACC = anterior cingulate cortex.
One common finding is that administration of nicotine (Domino et al., 2000b; Stapleton et al., 2003b) or cigarette smoking (Yamamoto et al., 2003) during scanning results in decreased global brain activity. Similarly, smokers who smoke ad lib prior to SPECT scanning (including the morning of the scan) have decreased global brain activity compared to former smokers and non-smokers (Rourke et al., 1997). These findings are generally supported by studies using transcranial Doppler ultrasound or the Xe 133 inhalation method to measure responses to smoking, with some (Cruickshank et al., 1989; Kubota et al., 1983, 1987; Rogers et al., 1983), but not all (Kodaira et al., 1993; Terborg et al., 2002), studies showing diminished cerebral blood flow.
A large (n = 86) recent study (Fallon et al., 2004) further characterized this decreased global activity with nicotine administration. 18F-fluorodeoxyglucose (FDG) PET was performed while smokers and ex-smokers performed the Bushman aggression task (designed to elicit an aggressive state) and wearing either a 0, 3.5, or 21 mg nicotine patch. Smokers who were rated high on the personality trait hostility had widespread cerebral metabolic decreases while wearing the 21 mg patch and performing the aggression task. Low hostility smokers did not have these changes during PET, suggesting that personality profile may determine which smokers have global metabolic decreases in response to nicotine.
In studies examining regional activity responses to nicotine or smoking, the most common findings are relative increases in activity in the prefrontal cortex (including the dorsolateral prefrontal cortex, and inferior frontal, medial frontal, and orbitofrontal gyri) (Domino et al., 2000b; Rose et al., 2003; Stein et al., 1998), thalamus (Domino et al., 2000a; Domino et al., 2000b; London et al., 1988a,b; Stein et al., 1998; Zubieta et al., 2001), and visual system (Domino et al., 2000a; Domino et al., 2000b; London et al., 1988a,b) (Jed Rose, Personal Communication). Additionally, a Xe 133 inhalation study reported increases in frontal lobe and thalamic blood flow in smokers who smoked a cigarette (Nakamura et al., 2000). The human studies here examined cigarette smokers, while the animal studies here used non-dependent rats, with strong concordance of findings between these sets of studies. Functional brain imaging studies of nicotine or cigarette administration to human non-smokers have not yet been reported, and would be important for a more complete understanding of the effects of tobacco on brain activity. While this group of studies demonstrate specific regional activation with nicotine or smoking, they also imply activation of cortico-basal ganglia-thalamic brain circuits (Alexander et al., 1990) that mediate the subjective effects of smoking (see Section 4).
Since regional activity was normalized to whole brain activity in at least some of these studies, and whole brain activity has been found to decrease with nicotine or cigarette administration (cited above), the regional findings presented here may represent either increased regional activity, or possibly, less of a decrease in regional activity than in other brain areas. Regional decreases in activity are generally not seen with nicotine or cigarette administration, though at least two studies found relatively decreased activity in the left (Rose et al., 2003) and right (Zubieta et al., 2001) amygdala.
2.2. Effect of nicotine on brain activation during cognitive tasks
The most commonly replicated cognitive effect of nicotine administration is improved performance on tasks that require vigilant attention in nicotine-dependent smokers (Newhouse et al., 2004). Nicotine administration also has been reported to improve reaction time (regardless of smoking status) as well (Ernst et al., 2001a). Consistent with these findings are studies which demonstrate that acute abstinence from smoking (within 12 h) results in slowed response times (Bell et al., 1999; Gross et al., 1993; Thompson et al., 2002).
In examining brain mediation of the cognitive effects of smoking, several groups have performed functional imaging studies in subjects performing cognitive tasks during administration of nicotine (compared to a control condition) (Table 2). For most of these studies, subjects performed a cognitive task that involved visual recognition and working memory, such as the n-back task. Results of these studies have been somewhat mixed, showing both decreased (Ernst et al., 2001b; Ghatan et al., 1998) and increased (Jacobsen et al., 2004; Kumari et al., 2003) ACC activation in response to nicotine administration while performing the task. Brain activation responses to nicotine during cognitive tasks have been more consistent in other brain areas such as the thalamus (Jacobsen et al., 2004; Lawrence et al., 2002) and visual cortex (Ghatan et al., 1998; Lawrence et al., 2002), while nicotine had no effect on the visual cortex during photic stimulation (Jacobsen et al., 2002). This last finding indicates that nicotine activates the visual cortex only during demanding visual tasks, rather than simple stimulation.
Table 2.
Functional brain imaging studies of nicotine or cigarette administration during cognitive tasks/stimulation
| Authors | Subjects | Method/task | Intervention | Effect of nicotine during task |
|---|---|---|---|---|
| Ghatan PH et al (Ghatan et al., 1998) |
12 smokers; 6 non- smokers |
15O-butanol PET/ computerized maze |
IV nic infusion versus abstinence |
↓ACC and cerebellum; ↑ occ ctx |
| Ernst et al (Ernst et al.. 2001b) | 11 smokers; 11 former smokers |
15O-PET/2-back | 2 pieces of 2 mg nic gum vs. placebo gum |
↓ACC and PFC activation in smokers |
| Jacobsen et al (Jacobsen et al., 2002) | 9 smokers | fMRI/photic stimulation | IV nic 10 mcg/kg vs. saline |
No effect on visual cortex |
| Lawrence et al (Lawrence et al., 2002) | 15 smokers | fMRI/rapid visual information-processing |
21 mg nic vs. placebo patch |
↑ parietal and occipital ctx,, thal, caudate |
| Kumari et al (Kumari et al., 2003) | 11 non-smoking men | fMRI/n-back | SC nic (1 mg) vs. saline | ↑ ACC, superior frontal ctx, superior parietal ctx |
| Jacobsen et al (Jacobsen et al., 2004) | 13 schizophrenic smokers; 13 smokers |
fMRI/n-back | 28 or 35 mg nic vs. placebo patch |
↑ ACC and bilateral thal activation (schizophrenic > non- schizophrenic) |
PET = positron emission tomography; IV = intravenous; nic = nicotine; ACC = anterior cingulate cortex; occ ctx = occipital cortex; PFC = prefrontal cortex; fMRI = functional magnetic resonance imaging; thal = thalamus.
2.3. Brain dopamine responses to nicotine and smoking
A common pathway for the positive reinforcement associated with most, if not all, addictive drugs is the brain dopamine (DA) system (Koob, 1992; Leshner and Koob, 1999). Laboratory animal studies demonstrate that DA release in the ventral striatum (VST)/nucleus accumbens (NAc) underlies the reinforcing properties of nicotine (Koob, 1992; Leshner and Koob, 1999). Microdialysis (Damsma et al., 1989; Di Chiara and Imperato, 1988; Pontieri et al., 1996; Sziraki et al., 2001) and lesion (Corrigall et al., 1992) studies in rats indicate that nicotine-induced DA release is strongest in this region, and is more robust than the DA release found in associated structures receiving dopaminergic input, such as the dorsal striatum (Di Chiara and Imperato, 1988). These studies generally used nicotine dosages that simulated human cigarette smoking. Acute exposure to cigarette smoke and nicotine has been found to up-regulate dopamine transporter mRNA in the ventral tegmental area (VTA) and substantia nigra (Li et al., 2004), and chronic exposure to cigarette smoke, more so than chronic nicotine alone, has also been found to up-regulate D1 and D2 receptor mRNA in the VST (Bahk et al., 2002). Additionally, many in vitro studies of the VST have reported DA release in response to nicotine (Connelly and Littleton, 1983; Marien et al., 1983; Rowell et al., 1987; Sakurai et al., 1982; Westfall et al., 1983).
Functional brain imaging studies of the DA system (Table 3) corroborate and expand upon these laboratory studies. Striatal DA release in response to a nicotine or cigarette challenge has been demonstrated repeatedly in both non-human primates and humans (Brody et al., 2004a; Dewey et al., 1999; Marenco et al., 2004; Tsukada et al., 2002), with the majority of these studies using PET and the radiotracer 11C-raclopride (a relatively specific D2 receptor binder) to demonstrate DA release through radiotracer displacement. These studies have reported a wide range of DA concentration change. In two studies that examined the question directly (Marenco et al., 2004; Tsukada et al., 2002), nicotine was found to result in less radiotracer displacement than amphetamine, while it has also been reported that nicotine-induced DA release is comparable in magnitude to that induced by other addictive drugs (Pontieri et al., 1996). In addition, an association between 11C-raclopride displacement and the hedonic effects of smoking (defined as elation and euphoria) has been demonstrated (Barrett et al., 2004), though this study did not find an overall difference between the smoking and non-smoking conditions. Thus, while the majority of studies do provide evidence for nicotine/smoking-induced DA release, there are disparities between studies in the extent of human smoking-induced DA release, leaving this issue currently unresolved. Disparities between these studies may be due to differences in methodology (e.g., nicotine administration versus cigarette smoking) and/or technical complexities in performing such studies. (As an aside, effects of smoking on dopamine projections to the prefrontal cortex (Goldman-Rakic et al., 1989) have not yet been reported with functional brain imaging.)
Table 3.
Functional imaging studies of the effects of nicotine or cigarette smoking on the dopamine (DA) system
| Authors | Subjects | Method | Intervention | Results/conclusions |
|---|---|---|---|---|
| Dewey et al (Dewey et al., 1999) | 16 baboons |
11C-raclopride PET (double bolus) |
IV nic (0.3 mg) | ↓DV tracer (indicating ↑ DA concentration) in Nac |
| Dagher et al (Dagher et al., 2001) | 11 smokers; 18 non- smokers |
11C-SCH 23390 PET | ↓BP in smokers (indicating ↓D1 receptor density) in ventral striatum |
|
| Tsukada et al (Tsukada et al., 2002) | 4 Macada mulatto monkeys |
11C-raclopride PET (B/I) | IV nic (B/I) | Slight ↓BP (indicating ↑ DA concentration) in anesthetized, but not conscious monkeys, in dorsal striatum |
| Salokangas et al (Salokangas et al., 2000b) |
9 smokers; 10 non- smokers |
18F-DOPA PET | ↑ uptake (indicating ↑ DA activity) in cd and Put of smokers |
|
| Krause et al (Krause et al., 2002) | 11 smoks w/ADHD; 11 non-smok w/ADHD |
[99mTc]TRODAT SPECT |
↓ DAT (striatal) in smokers |
|
| Staley et al (Staley et al., 2001) | 21 smokers; 21 non- smokers |
[123I]beta-CIT SPECT | No overall binding difference between smokers and non- smokers; ↑ brainstem 5- HT transporters in male smokers |
|
| Marenco et al (Marenco et al., 2004) | 5 rhesus monkeys |
11C-raclopride PET (double bolus & B/I) |
IV nic (0.01 to 0.06 mg/ kg) |
↓ BP (indicating ↑DA concentration) in basal ganglia with nic administration |
| Brody et al (Brody et al., 2004a) | 20 smokers | 11C-raclopride PET (B/I) | Single cigarette versus no smoking |
↓ BP (indicating ↑ DA concentration) in smoking, but not no smoking, condition in L ventral cd and put |
| Barrett et al (Barrett et al., 2004) | 10 smokers |
11C-raclopride PET (double bolus) |
Smoking every 12 minutes versus no smoking |
↓BP correlated with hedonic response to smoking in cd and posterior put |
PET = positron emission tomography; IV = intravenous; nic = nicotine; DV = volume of distribution; DA = dopamine; BP = binding potential; B/I = bolus-plus-infusion; cd = caudate; put = putamen; SPECT = single photon emission computed tomography; DAT = dopamine transporter; ADHD = attention deficit hyperactivity disorder; beta-CIT = (2 beta-carbomethoxy-3 beta-(4-iodophenyl)-tropane); 5-HT = serotonin.
Nicotine-induced DA release in the NAc has been reported to be mediated by stimulation of nicotinic acetylcholine receptors (nAChRs) on cells of the ventral tegmental area (VTA) that project to the NAc rather than by nicotinic receptors within the NAc itself (Nisell et al., 1994). Lesioning of mesolimbic VTA neurons projecting to the NAc leads to decreased nicotine self-administration (Corrigall et al., 1992; Lanca et al., 2000). Additionally, the effects of nicotine on the dopaminergic system appear to be modulated by glutamatergic and GABAergic neurons (Picciotto and Corrigall, 2002), with nicotine stimulation of gluatamatergic tracts from the prefrontal cortex to the VTA leading to increased DA neuron firing (Kenny and Markou, 2001) and GABA agonism leading to a dampening of DA neuron responses (Cousins et al., 2002). Recent work indicates that nicotine administration causes prolonged depression of GABAergic firing leading to relatively greater excitatory (glutamatergic) input into the mesolimbic DA system and increased DA neuron firing (Mansvelder et al., 2002).
Other functional imaging studies of the DA system have reported decreased D1 receptor density (Dagher et al., 2001), increased 18F-DOPA uptake (a marker for increased DA turnover) (Salokangas et al., 2000a), and both decreased (Krause et al., 2002) and no alterations (Staley et al., 2001) in dopamine transporter binding in smokers.
To summarize these studies of the DA system, there is extensive evidence that nicotine administration and smoking result in activation of the brain DA mesolimbic pathway, resulting in increased DA release and turnover in the VST/NAc. Because dopaminergic input to the NAc modulates neurotransmission through cortico-basal ganglia-thalamic circuitry (Haber and Fudge, 1997), smoking-induced increases in DA concentration may explain some of the clinical effects of smoking as discussed below (Section 4).
2.4. Functional imaging of nicotinic acetylcholine receptors
Because stimulation of nicotinic acetylcholine receptors (nAChRs) is intimately linked with effects of smoking, a longstanding and still developing area of research is the labeling of nAChRs using functional brain imaging. Nicotinic acetylcholine receptors are ligand-gated ion channels consisting of α and β subunits (Court et al., 2000; Hogg et al., 2003). At least twelve nAChRs have been identified with the heteromeric α4β2 being the most common subtype in the brain and the homomeric α7 being the next most common. Post-mortem (Benwell et al., 1988; Breese et al., 1997) and laboratory (Yates et al., 1995) studies demonstrate that smokers have widespread up-regulation of nAChRs, likely related to desensitization of these receptors from nicotine exposure. (Many animal studies also demonstrate up-regulation of nAChRs in response to chronic nicotine administration) e.g. (Pauly et al., 1996; Shoaib et al., 1997; Zhang et al., 2002). Thus, nAChRs are a natural target for tracer development in the pursuit of a greater understanding of tobacco dependence and other illnesses with abnormal nAChR levels.
Animal research demonstrates that nicotine binds to nAChRs in the brain to mediate a variety of behavioral states (Lukas, 1998; Paterson and Nordberg, 2000)such as heightened arousal and improved reaction time and psychomotor function (Paterson and Nordberg, 2000). Nicotine administration also produces reward through DA release in the NAc, at least in part through stimulation of nAChRs in the ventral tegmental area (Blaha et al., 1996; Corrigall et al., 1994; Nisell et al., 1994; Yeomans and Baptista, 1997; Yoshida et al., 1993). Nicotinic acetylcholine receptors are widespread throughout the brain, with a rank order distribution of nAChR density being: thalamus > basal ganglia > cerebral cortex > hippocampus > cerebellum (Broussolle et al., 1989; Cimino et al., 1992; Clarke et al., 1984; Davila-Garcia et al., 1999; Dávila-García et al., 1997; London et al., 1995, 1985; Pabreza et al., 1991; Pauly et al., 1989; Perry and Kellar, 1995; Valette et al., 1998; Villemagne et al., 1997).
Innovative researchers have developed tracers for the nAChR in recent years, with labeled A-85380 (3-(2(S)-azetidinylmethoxy) pyridine) (Koren et al., 1998) compounds having the most widespread use. Radiolabeling of A-85380 was a major advance in imaging nAChRs, because administration of radiolabeled nicotine (used for previous imaging studies) results in high non-specific binding and short drug–receptor interaction times (Sihver et al., 2000). In recent years, 2-[18F]F-A-85380 or simply 2-FA and related compounds (Chefer et al., 1999; Horti et al., 1998; Koren et al., 1998) have been developed for PET imaging, and 5-[123/125I]iodo-A85380 has been used for SPECT imaging (Chefer et al., 1998; Horti et al., 1999; Mukhin et al., 2000) of α4β2 nAChRs.
Studies of non-human primates and humans have examined distributions of nAChRs with these new tracers, and found regional densities of these receptors similar to those in the animal work cited above (Chefer et al., 1999, 2003; Fujita et al., 2002; Fujita et al., 2003; Kimes et al., 2003; Valette et al., 1999). In initial human studies, no subjective or cardiovascular effects of 2-FA have been reported; however, studies of tobacco dependent subjects have not yet been published. Finally, two recent studies of baboons examined effects of nicotine or tobacco smoke on nAChR availability. In a 2-FA PET study (Valette et al., 2003), IV nicotine (0.6 mg), inhalation of tobacco smoke from one cigarette (0.9 mg nicotine), and IV nornicotine were all found to reduce the volume of distribution of the tracer by roughly 30–60% in the thalamus and putamen at 80 min, and this reduction of 2-FA binding was relatively long-lived (up to 6 h). Similarly, a 50% reduction in nAChR availability was found with IV nicotine administration to baboons using an epibatidine analog and PET scanning (Ding et al., 2000). Taken together, these studies demonstrate that radiotracers for nAChRs can be administered safely to measure nAChR densities, and that nicotine and smoking substantially decrease α4β2 nAChR availability.
2.5. Glutamatergic (and other) effects of nicotine/cigarette smoking
Recent autoradiography studies of rodents are determining effects of nicotine/smoking in brain systems that may be activated by nAChR stimulation. For example, in response to nicotine, glutamate release has been demonstrated in the prelimbic prefrontal cortex (Gioanni et al., 1999), and glutamate and aspartate release have been demonstrated in the VTA (Schilstrom et al., 2000). The finding of nAChR-induced glutamate release in the prefrontal cortex has also been demonstrated by measuring spontaneous excitatory postsynaptic currents (Lambe et al., 2003). Importantly, one of these studies (Gioanni et al., 1999) also demonstrated that nicotine administration facilitates thalamo-cortical neurotransmission through stimulation of nAChRs on glutamatergic neurons.
Other autoradiography studies of rats have demonstrated that chronic administration of nicotine increases glucose transporter (Glut1 and Glut3) densities in an array of brain areas (Duelli et al., 1998) and that chronically administered low dose nicotine is protective against neurodegenerative agents in the striatum (a model for Parkinson’s Disease) (Ryan et al., 2001).
3. Brain function responses to chronic nicotine administration and cigarette smoking
3.1. Functional brain imaging of cigarette craving
Turning to brain imaging of tobacco/nicotine dependence, chronic cigarette smokers experience craving for cigarettes (urge to smoke) within minutes after the last cigarette, and the intensity of craving rises over the next 3–6 h (Jarvik et al., 2000; Schuh and Stitzer, 1995). Cigarette-related cues have been shown to reliably enhance craving during this period, when compared to neutral cues (Carter and Tiffany, 1999).
Two recent studies used a cigarette versus neutral cue paradigm paired with functional imaging to evaluate brain mediation of cigarette craving. In one study (Due et al., 2002), 6 smokers and 6 non-smokers underwent event-related fMRI when presented with smoking images (color photographs) compared with neutral images, for 4 s each. For the smoker group, craving increased during the testing session and exposure to smoking images resulted in activation of mesolimbic (right posterior amygdala, posterior hippocampus, ventral tegmental area, and medial thalamus) and visuospatial cortical attention (bilateral prefrontal and parietal cortex and right fusiform gyrus) circuitry, while the non-smoker group did not have these changes. In the second study (Brody et al., 2002), 20 smokers and 20 non-smokers underwent two FDG-PET sessions. For one PET session, subjects held a cigarette and watched a cigarette-related video, while for the other, subjects held a pen and watched a nature video (randomized order) during the 30-min uptake period of FDG. When presented with smoking-related (compared to neutral) cues, smokers had higher regional metabolism in bilateral anterior cingulate cortex (ACC), left orbitofrontal cortex (OFC), and left anterior temporal lobe. Change in craving scores was also positively correlated with change in metabolism in the OFC, dorsolateral prefrontal cortex, and anterior insula bilaterally.
Taken together, these studies of cigarette craving indicate that immediate responses to visual smoking-related cues (fMRI study) activate the brain reward system, limbic regions, and the visual processing system, while longer exposure to cues (FDG-PET study) leads to activation of the ACC, which mediates anxiety, alertness, and arousal (Chua et al., 1999; Critchley et al., 2001; Kimbrell et al., 1999; Naito et al., 2000; Rauch et al., 1999) and the OFC, which functions in part as a secondary processing center for sensory information (Rolls et al., 1998; Rolls and Baylis, 1994).
In a related preliminary study, seventeen smokers underwent the same FDG-PET craving versus neutral cue protocol as in the second study of craving listed above (Brody et al., 2002) after treatment with a standard course of bupropion HCl (tapered up to 150 mg per oral twice a day for a mean 5.6 weeks). This group of treated subjects had a significant reduction in smoking levels from pre- to post-treatment (mean 27.1 cigs/d pre-treatment to a mean of 3.7 cigs/d post-treatment). Bupropion-treated smokers also had reduced cigarette cue-induced craving and diminished ACC activation when presented with cigarette-related cues, compared to untreated smokers (Brody et al., 2004b). This diminished ACC activation was due to elevated baseline normalized ACC activity in treated smokers, giving an indication that bupropion treatment of smokers increases resting ACC metabolism.
3.2. Functional brain imaging of cigarette withdrawal
Brain activity changes (measured with fMRI) during cigarette withdrawal were recently reported for nicotine-dependent rats (Shoaib et al., 2004). In this study, subcutaneous mecamylamine (1 mg/kg), a nicotine receptor antagonist, was administered to precipitate withdrawal during scanning, and this state was compared to a control state after subcutaneous saline administration. After subcutaneous mecamylamine, nicotine dependent rats had bilateral increases in nucleus accumbens activity compared to the control state.
3.3. Monoamine oxidase function in smokers
Fowler and colleagues have performed a series of elegant studies demonstrating decreases in monoamine oxidase (MAO) A and B activity in cigarette smokers using the PET tracers [11C]clorgyline (Fowler et al., 1996b) and ([11C]L-deprenyl-D2) (Fowler et al., 1996a, 1998b), respectively. When compared to former smokers and non-smokers, average reductions for current smokers are 30% and 40% for MAO A and B (Fowler et al., 2003a). These reductions are the result of chronic smoking behavior rather than a single administration of intravenous nicotine (Fowler et al., 1998a) or smoking a single cigarette (Fowler et al., 1999, 2000), and are less than those seen with antidepressant MAO inhibitors (Fowler et al., 1994; Fowler et al., 1996b). Additionally, a human post-mortem study of chronic smokers demonstrated a modest reduction in MAO A binding that did not reach statistical significance (Klimek et al., 2001). Peripheral MAO B is also reduced in cigarette smokers (Fowler et al., 2003b).
MAO participates in the catabolism of dopamine, norepinephrine, and serotonin (Berlin and Anthenelli, 2001; Fowler et al., 2003a), and it has been postulated that some of the clinical effects of smoking are due to MAO inhibition, leading to decreases in monoamine breakdown with a subsequent increase in monoamine availability (Berlin and Anthenelli, 2001). Thus, smoking may enhance DA availability and the rewarding properties of smoking both through DA release (as described above) and MAO inhibition. Smoking may also alter mood and anxiety through MAO inhibition effects on norepinephrine and serotonin availability and turnover. Comprehensive reviews of the role of MAO in tobacco dependence have recently been published (Berlin and Anthenelli, 2001; Fowler et al., 2003a).
4. Discussion: functional neuroanatomy of tobacco use and dependence
Both acute and chronic effects of nicotine/cigarette exposure have been elucidated with functional brain imaging. Replicated responses to acute administration of nicotine/smoking include: a reduction in global brain activity (perhaps most prominently in smokers with high levels of hostility as a personality trait); activation of the prefrontal cortex, thalamus, and visual system; activation of the thalamus and visual cortex (and possibly ACC) during visual cognitive tasks; and increased DA concentration in the ventral striatum/NAc. Replicated responses to chronic nicotine/cigarette exposure include decreased MAO A and B activity and a substantial reduction in α4β2 nAChR availability in the thalamus and putamen (accompanied by an overall up-regulation of these receptors).
This group of findings demonstrates a number of ways in which smoking might enhance neurotransmission through cortico-basal ganglia-thalamic circuits (Alexander et al., 1990) (in addition to demonstrating direct effects of chronic nicotine exposure on nAChR availability) (Fig. 1). Given that the thalamus (Groenewegen et al., 1999; Herrero et al., 2002; Sommer, 2003) and ventral striatum/Nac (Groenewegen et al., 1999; Herrero et al., 2002) function as relay centers for information and for paralimbic and motor processing in the brain, the net effect of smoking may be to enhance neurotransmission along cortico-basal ganglia-thalamic loops originating in prefrontal and paralimbic cortices. Neurotransmission through these circuits may be stimulated directly by the interconnected (Sherman, 2001; Sillito and Jones, 2002) nAChR-rich thalamus and visual systems, and/or indirectly through effects on MAO inhibition and DA release in the ventral striatum/NAc (as well as through nicotine stimulation of excitatory glutamatergic input to the dopamine system (Mansvelder et al., 2002)). In the thalamus, for example, nicotine has direct agonist action on excitatory thalamocortical projection neurons and local circuit neurons, although nicotine also stimulates GABAergic interneurons, so that the relationship between nicotine stimulation and thalamocortical stimulation may be complex (Clarke, 2004). There is mixed evidence as to whether or not nicotine stimulates corticothalamic neurons (Clarke, 2004).
Fig. 1.
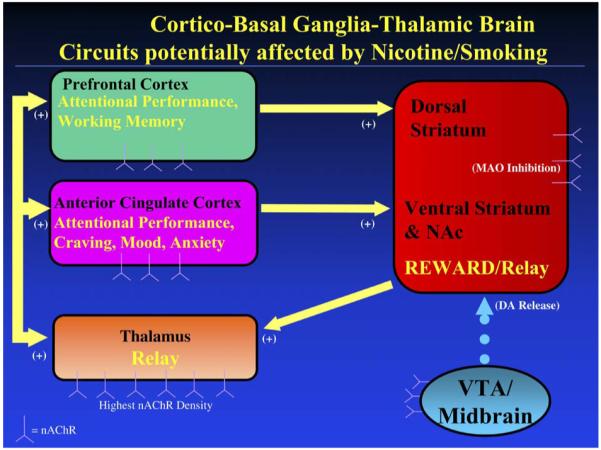
Simplified representation of cortico-basal ganglia-thalamic brain circuitry that mediates effects of nicotine/smoking on attentional control, craving, mood and anxiety. Potential targets for nicotine/smoking to enhance attention (and improve craving, mood, and anxiety) include: (1) direct stimulation of nicotinic acetylcholine receptors (nAChRs) in cortex, (2) stimulation of the nAChR-rich thalamus and basal ganglia (which function as relay stations for this circuitry), (3) activation of dopaminergic mesolimbic reward pathways originating in the ventral tegmental area and projecting to the striatum, and (4) monoamine oxidase (MAO) inhibition in the basal ganglia. NAc = nucleus accumbens; VTA = ventral tegmental area.
Enhancement of neurotransmission through prefrontal and paralimbic cortico-basal ganglia-thalamic circuits may account for the most commonly reported cognitive effect of cigarette smoking, namely improved attentional performance (Newhouse et al., 2004), and also related effects, such as improvements in reaction times (Hatsukami et al., 1989; Pritchard et al., 1992; Shiffman et al., 1995), arousal (Parrott and Kaye, 1999), motivation (Powell et al., 2002), and sustained attention (Rusted et al., 2000). Prefrontal (including both dorsolateral and ventrolateral) (Duncan and Owen, 2000; Rees and Lavie, 2001; Smith and Jonides, 1999) and ACC (Carter et al., 1999; Duncan and Owen, 2000; Peterson et al., 1999; Smith and Jonides, 1999) cortices are reported to activate during attentional control tasks (especially visuospatial tasks) (Pessoa et al., 2003). Cigarette smoking may enhance attentional control through direct stimulation of nAChRs within these structures or perhaps through subcortical stimulation of nAChRs in the thalamus and via DA release and/or MAO inhibition in the basal ganglia.
In addition to improvement in attention, smoking improves withdrawal symptoms, such as depressed mood, anxiety, and irritability in tobacco dependent smokers (Cohen et al., 1991; Parrott, 2003), and all of these effects depend (at least in part) on the expectations of the smoker (Perkins et al., 2003). Though nicotine administration generally results in increased activity along prefrontal and paralimbic brain circuits, it is interesting that both increased and decreased ACC activation during cognitive task performance has been reported (see Section 2.2). ACC activity has been associated with anxiety and mood, with increased activity being associated with greater anxiety (Chua et al., 1999; Kimbrell et al., 1999) and decreased activity being associated with depressed mood (Drevets et al., 1997). This combination of findings suggests a potential interaction between expectation of the effects of smoking (e.g. mood improvement, anxiety reduction, or decreased irritability) and direction of ACC activity change during cognitively demanding tasks. Perhaps smokers who expect to and do have anxiety alleviation from smoking have deactivation or decreased activation of the ACC while performing cognitive tasks, while those who expect to and do experience mood improvement from smoking have increased activation of the ACC.
In addition to these primary effects of nicotine and smoking, other functional imaging studies reviewed here focus on smoking-related states, such as cue-induced cigarette craving. Such studies are part of a large body of literature examining cue-induced craving for addictive drugs. Studies specific for cigarette cues/craving reveal that exposure to visual cigarette cues immediately activates mesolimbic (ventral tegmental area, amygdala, and hippocampus) and visuospatial cortical attention areas of the brain, and acutely (over a 30 min time period) activate paralimbic regions (ACC and OFC), and that this cue-induced activation may be diminished by a course of bupropion treatment. These results are similar to those of functional imaging studies for drugs other than tobacco (Goldstein and Volkow, 2002; Miller and Goldsmith, 2001). and it has been posited that at least some of the activations seen with cigarette-related cues (cortical attention areas and OFC) are associated with an expectation of smoking in the non-treatment seeking subjects who participated in these studies (Wilson et al., 2004).
In summary, functional brain imaging studies of nicotine/cigarette smoking have demonstrated a link between nicotine/cigarette administration and brain circuitry that mediates visuospatial attentional processing and withdrawal symptoms. Future studies utilizing newer PET tracers and enhanced MRI techniques will undoubtedly further elucidate the brain mediation of tobacco dependence, and may accelerate the development of targeted smoking cessation therapies.
Acknowledgements
The author would like to thank Sanjaya Saxena, M.D., for his helpful comments on the manuscript. This work was supported by a Department of Veterans Affairs Type I Merit Review Award, the Tobacco-Related Disease Research Program (11RT-0024), the National Institute on Drug Abuse (R01 DA15059), and a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamo-cortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–46. [PubMed] [Google Scholar]
- Bahk JY, Li SP, Park MS, Kim MO. Dopamine D-1 and D-2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:1095–104. doi: 10.1016/s0278-5846(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Baker RR, Massey ED, Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food and Chemical Toxicology. 2004;42(Suppl):S53–83. doi: 10.1016/j.fct.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Balluz L, Ahluwalia IB, Murphy W, Mokdad A, Giles W, Harris VB. Surveillance for certain health behaviors among selected local areas–United States, Behavioral Risk Factor Surveillance System, 2002. MMWR Surveill Summary. 2004;53:1–100. [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [(11)C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Bartal M. Health effects of tobacco use and exposure. Monaldi Archives of Chest Disease. 2001;56:545–54. [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tobacco Research. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. Journal of Neurochemistry. 1988;50:1243–7. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. International Journal of Neuropsychopharmacology. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. Journal of Neuroscience. 1996;16:714–22. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. Journal of Pharmacology and Experimental Therapeutics. 1997;282:7–13. [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004a;161:1211–8. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Research Neuroimaging. 2004b;130:269–81. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML, Lee GS, Saxena S, Baxter LR, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Broussolle EP, Wong D, Fanelli RJ, London ED. In vivo specific binding of [3H]-nicotine in the mouse brain. Life Science. 1989;44:1123–32. doi: 10.1016/0024-3205(89)90340-8. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Koren AO, Gündrisch D, Links JM, Kurian V, Dannals RF, Mukhin AG, London ED. 2-[18F]F-A-83580: a PET radioligand for a4b2 nicotinic acetylcholine receptors. Neuroreport. 1999;10:2715–21. doi: 10.1097/00001756-199909090-00005. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Lee K, Koren A, Jones DW, Gorey J, Links JM, Mukhin AG, Weinberger DR, London ED. In vivo imaging of brain nicotinic receptors with 5-[123I]iodo-A-85380 using single photon emission computed tomography. Life Science. 1998;63:PL355–60. doi: 10.1016/s0024-3205(98)00514-1. [DOI] [PubMed] [Google Scholar]
- Chefer SI, London ED, Koren AO, Pavlova OA, Kurian V, Kimes AS, Horti AG, Mukhin AG. Graphical analysis of 2-[F-18]FA binding to nicotinic acetylcholine receptors in rhesus monkey brain. Synapse. 2003;48:25–34. doi: 10.1002/syn.10180. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–71. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Cimino M, Marini P, Fornasari D, Cattabeni F, Clementi F. Distribution of nicotinic receptors in cynomolgus monkey brain and ganglia: localization of alpha 3 subunit mRNA, alpha-bungarotoxin and nicotine binding sites. Neuroscience. 1992;51:77–86. doi: 10.1016/0306-4522(92)90472-e. [DOI] [PubMed] [Google Scholar]
- Clarke PBS. Nicotinic modulation of thalamocortical neurotransmission. Acetylcholine in the Cerebral Cortex. 2004;145:253–60. doi: 10.1016/S0079-6123(03)45017-6. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Pert C, Pert A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Research. 1984;323:390–5. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Cohen C, Pickworth WB, Henningfield JE. Cigarette smoking and addiction. Clinics in Chest Medicine. 1991;12:701–10. [PubMed] [Google Scholar]
- Connelly MS, Littleton JM. Lack of stereoselectivity in ability of nicotine to release dopamine from rat synaptosomal preparations. Journal of Neurochemistry. 1983;41:1297–302. doi: 10.1111/j.1471-4159.1983.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653:278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. Journal of Chemical Neuroanatomy. 2000;20:281–98. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug and Alcohol Dependence. 2002;65:209–20. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuroimage. 2001;13:S392. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Cruickshank JM, Neildwyer G, Dorrance DE, Hayes Y, Patel S. Acute Effects of Smoking on Blood-Pressure and Cerebral Blood-Flow. Journal of Human Hypertension. 1989;3:443–9. [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JAD, Gunn RN, Clarke PBS, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. European Journal of Pharmacology. 1989;168:363–8. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Davila-Garcia MI, Houghtling RA, Qasba SS, Kellar KJ. Nicotinic receptor binding sites in rat primary neuronal cells in culture: characterization and their regulation by chronic nicotine. Molecular Brain Research. 1999;66:14–23. doi: 10.1016/s0169-328x(98)00344-1. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio J, Perry D, Xiao Y, Horti A, London E, Dannals RF, Kellar K. [125I]IPH, an epibatidine analog, binds with high affinity to neuronal nicotinic cholinergic receptors. Journal of Pharmacology and Experimental Therapeutics. 1997;282:445–51. [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CRJ. A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Volkow ND, Logan J, Garza V, Pappas N, King P, Fowler JS. Occupancy of brain nicotinic acetylcholine receptors by nicotine doses equivalent to those obtained when smoking a cigarette. Synapse. 2000;35:234–7. doi: 10.1002/(SICI)1098-2396(20000301)35:3<234::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000a;38:313–21. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, Cross DJ, Zubieta J. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000b;101:277–82. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duelli R, Staudt R, Grunwald F, Kuschinsky W. Increase of glucose transporter densities (Glut1 and Glut3) during chronic administration of nicotine in rat brain. Brain Research. 1998;782:36–42. doi: 10.1016/s0006-8993(97)01264-x. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001a;25:313–9. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2001b;98:4728–33. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Keator DB, Mbogori J, Turner J, Potkin SG. Hostility differentiates the brain metabolic effects of nicotine. Brain Research, Cognition Brain Research. 2004;18:142–8. doi: 10.1016/j.cogbrainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. US Department of Health and Human Services. Public Health Service; Rockville, MD: 2000. Treating Tobacco Use and Dependence. [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003a;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Pappas N, Ferrieri R, Shea C, Garza V, Xu YW, Schlyer D, Gatley SJ, Ding YS, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Low monoamine oxidase B in peripheral organs in smokers. Proceedings of the National Academy of Sciences of the United States of America. 2003b;100:11600–5. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Pappas N, King P, MacGregor R, Shea C, Garza V, Gatley SJ. An acute dose of nicotine does not inhibit MAO B in baboon brain in vivo. Life Sciences. 1998a;63:L19–23. doi: 10.1016/s0024-3205(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, Wolf AP, Pappas N, Alexoff D, Shea C. Slow recovery of human brain MAO B after L-deprenyl (Selegeline) withdrawal. Synapse. 1994;18:86–93. doi: 10.1002/syn.890180203. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379:733–6. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I. Neuropharmacological actions of cigarette smoke: Brain monoamine oxidase B (MAO B) inhibition. Journal of Addictive Diseases. 1998b;17:23–34. doi: 10.1300/J069v17n01_03. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proceedings of the National Academy of Sciences of the United States of America. 1996b;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Franceschi D, Logan J, Pappas N, Shea C, MacGregor RR, Garza V. Maintenance of brain monoamine oxidase B inhibition in smokers after overnight cigarette abstinence. American Journal of Psychiatry. 2000;157:1864–6. doi: 10.1176/appi.ajp.157.11.1864. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Franceschi D, Logan J, Pappas N, Shea C, MacGregor RR, Garza V. Smoking a single cigarette does not produce a measurable reduction in brain MAO B in non-smokers. Nicotine Tobacco Research. 1999;1:325–9. doi: 10.1080/14622299050011451. [DOI] [PubMed] [Google Scholar]
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco Control. 2003;12:424–30. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Ichise M, van Dyck CH, Zoghbi SS, Tamagnan G, Mukhin AG, Bozkurt A, Seneca N, Tipre D, DeNucci CC, Iida H, Vaupel DB, Horti AG, Koren AO, Kimes AS, London ED, Seibyl JP, Baldwin RM, Innis RB. Quantification of nicotinic acetylcholine receptors in human brain using [I-123]5-I-A-85380 SPET. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30:1620–9. doi: 10.1007/s00259-003-1320-0. [DOI] [PubMed] [Google Scholar]
- Fujita M, Seibyl JP, Vaupel DB, Tamagnan G, Early M, Zoghbi SS, Baldwin RM, Horti AG, Koren AO, Mukhin AG, Khan S, Bozkurt A, Kimes AS, London ED, Innis RB. Whole-body biodistribution, radiation absorbed dose, and brain SPET imaging with [123i]5-i-A-85380 in healthy human subjects. European Journal of Nuclear Medicine and Molecular Imaging. 2002;29:183–90. doi: 10.1007/s00259-001-0695-z. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–89. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepouse C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. European Journal of Neuroscience. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9015–9. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Galis-de Graaf Y, Smeets WJAJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. Journal of Chemical Neuroanatomy. 1999;16:167–85. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology (Berl) 1993;110:333–6. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Critical Reviews in Neurobiology. 1997;11:323–42. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. Journal of Substance Abuse. 1989;1:407–16. [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nervous System. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Reviews of Physiology Biochemistry and Pharmacology. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Holmes S, Zwar N, Jimenez-Ruiz CA, Ryan PJ, Browning D, Bergmann L, Johnston JA. Bupropion as an aid to smoking cessation: a review of real-life effectiveness. International Journal of Clinical Practice. 2004;58:285–91. doi: 10.1111/j.1368-5031.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- Horti AG, Koren AO, Lee KS, Mukhin AG, Vaupel DB, Kimes AS, Stratton M, London ED. Radiosynthesis and preliminary evaluation of 5-[123/125I]iodo-3-(2(S)-azetidinylmethoxy)pyridine: a radioligand for nicotinic acetylcholine receptors. Nuclear Medicine and Biology. 1999;26:175–82. doi: 10.1016/s0969-8051(98)00086-9. [DOI] [PubMed] [Google Scholar]
- Horti AG, Scheffel U, Koren AO, Ravert HT, Mathews WB, Musachio JL, Finley PA, London ED, Dannals RF. 2-[F-18]fluoro-A-85380, an in vivo tracer for the nicotinic acetylcholine receptors. Nuclear Medicine and Biology. 1998;25:599–603. doi: 10.1016/s0969-8051(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, Broughton JO, Fortmann SP, Leischow SJ, McKenna JP, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tobacco Research. 1999;1:169–74. doi: 10.1080/14622299050011281. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. NEJM. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biological Psychiatry. 2004;55:850–8. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magnetic Resonance Imaging. 2002;20:141–5. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000;66:553–8. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. NEJM. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology Biochemistry and Behavior. 2001;70:531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology. 1999;7:226–33. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, Strausberg L, Varady A. Nicotine patch and paroxetine for smoking cessation. Journal of Consulting and Clinical Psychology. 2000;68:883–9. [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, Repella JD, Benson BE, Willis MW, Herscovitch P, Post RM. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry. 1999;46:454–65. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB Journal. 2003;17:1331–3. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- Klimek V, Zhu MY, Dilley G, Konick L, Overholser JC, Meltzer HY, May WL, Stockmeier CA, Ordway GA. Effects of long-term cigarette smoking on the human locus coeruleus. Archives of General Psychiatry. 2001;58:821–7. doi: 10.1001/archpsyc.58.9.821. [DOI] [PubMed] [Google Scholar]
- Kodaira K, Fujishiro K, Wada T, Maie K, Satoi T, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S, Okamura T. A study on cerebral nicotine receptor distribution, blood flow, oxygen consumption, and other metabolic activities-a study on the effects of smoking on carotid and cerebral artery blood flow. Yakubutsu Seishin Kodo. 1993;13:157–65. [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koren AO, Horti AG, Mukhin AG, Gundisch D, Kimes AS, Dannals RF, London ED. 2-, 5-, and 6-halo-3-(2(S)-azetidinylmethoxy)pyridines: Synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling. Journal of Medicinal Chemistry. 1998;41:3690–8. doi: 10.1021/jm980170a. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K, Ackenheil M. Stimulant-like action of nicotine on striatal dopamine transporter in the brain of adults with attention deficit hyperactivity disorder. International Journal of Neuropsychopharmacology. 2002;5:111–3. doi: 10.1017/S1461145702002821. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Abe Y, Fujiwara T, Hatazawa J, Matsuzawa T. Effects of smoking on regional cerebral blood-flow in neurologically normal subjects. Stroke. 1983;14:720–4. doi: 10.1161/01.str.14.5.720. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Fujiwara T, Matsuzawa T. Effects of smoking on regional cerebral blood-flow in cerebral vascular-disease patients and normal subjects. Tohoku Journal of Experimental Medicine. 1987;151:261–8. doi: 10.1620/tjem.151.261. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–13. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–25. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–42. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–48. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Leistikow BN, Martin DC, Milano CE. Estimates of smoking-attributable deaths at ages 15-54, motherless or fatherless youths, and resulting Social Security costs in the United States in 1994. Preventive Medicine. 2000a;30:353–60. doi: 10.1006/pmed.2000.0657. [DOI] [PubMed] [Google Scholar]
- Leistikow BN, Martin DC, Milano CE. Fire injuries, disasters, and costs from cigarettes and cigarette lights: a global overview. Preventive Medicine. 2000b;31:91–9. doi: 10.1006/pmed.2000.0680. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proceedings of the Association of American Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Li SP, Kim KY, Kim JH, Kim JH, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neuroscience Letters. 2004;363:29–32. doi: 10.1016/j.neulet.2004.03.053. [DOI] [PubMed] [Google Scholar]
- London ED, Connolly RJ, Szikszay M, Wamsley JK, Dam M. Effects of nicotine on local cerebral glucose-utilization in the rat. Journal of Neuroscience. 1988a;8:3920–8. doi: 10.1523/JNEUROSCI.08-10-03920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Dam M, Fanelli RJ. Nicotine enhances cerebral glucose utilization in central components of the rat visual system. Brain Research Bulletin. 1988b;20:381–5. doi: 10.1016/0361-9230(88)90067-6. [DOI] [PubMed] [Google Scholar]
- London ED, Scheffel U, Kimes AS, Kellar KJ. In vivo labeling of nicotinic acetylcholine receptors in brain with [3H]epibatidine. European Journal of Pharmacology. 1995;278:R1–2. doi: 10.1016/0014-2999(95)00178-n. [DOI] [PubMed] [Google Scholar]
- London ED, Waller SB, Wamsley JK. Autoradiographic localization of [3H]nicotine binding sites in the rat brain. Neuroscience Letters. 1985;53:179–84. doi: 10.1016/0304-3940(85)90182-x. [DOI] [PubMed] [Google Scholar]
- Lukas RJ. Neuronal nicotinic acetylcholine receptors. In: Barrantes FJ, editor. The nicotinic acetylcholine receptor: current views and future trends. R.G. Landes Company; Georgetown: 1998. pp. 145–73. [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [C-11]raclopride PET. Neuropsychopharmacology. 2004;29:259–68. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Marenco T, Bernstein S, Cumming P, Clarke PBS. Effects of nicotine and chlorisondamine on cerebral glucose utilization in immobilized and freely-moving rats. British Journal of Pharmacology. 2000;129:147–55. doi: 10.1038/sj.bjp.0703005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien M, Brien J, Jhamandas K. Regional release of [3H]dopamine from rat brain in vitro: effects of opioids on release induced by potassium, nicotine, and L-glutamic acid. Candian Journal of Physiology and Pharmacology. 1983;61:43–60. doi: 10.1139/y83-005. [DOI] [PubMed] [Google Scholar]
- Miller NS, Goldsmith RJ. Craving for alcohol and drugs in animals and humans: biology and behavior. Journal of Addictive Diseases. 2001;20:87–104. doi: 10.1300/J069v20n03_08. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-iodo-A-85380, an alpha 4 beta 2 subtype-selective ligand for nicotinic acetylcholine receptors. Molecular Pharmacology. 2000;57:642–9. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. Journal of Neurophysiology. 2000;83:1701–9. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Tanaka A, Nomoto Y, Ueno Y, Nakayama Y. Activation of fronto-limbic system in the human brain by cigarette smoking: evaluated by a CBF measurement. Keio Journal of Medicine. 2000;49(Suppl 1):A122–4. [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Pabreza LA, Dhawan S, Kellar KJ. [3H]Cytisine binding to nicotinic cholinergic receptors in brain. Molecular Pharmacology. 1991;39:9–12. [PubMed] [Google Scholar]
- Parrott AC. Cigarette-derived nicotine is not a medicine. World Journal of Biological Psychiatry. 2003;4:49–55. doi: 10.3109/15622970309167951. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behavioural Pharmacology. 1999;10:639–46. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Progress in Neurobiology. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. European Neuropsychopharmacology. 2002;12:495–501. doi: 10.1016/s0924-977x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. Journal of Pharmacology and Experimental Therapeutics. 1996;278:361–9. [PubMed] [Google Scholar]
- Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Research Bulletin. 1989;22:453–9. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tobacco Research. 2003;5:695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Perry DC, Kellar KJ. [3H]Epibatidine labels nicotinic receptors in rat brain: An autoradiographic study. Journal of Pharmacology and Experimental Therapeutics. 1995;285:1030–4. [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. Journal of Neuroscience. 2003;23:3990–8. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang HP, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–58. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. Journal of Neuroscience. 2002;22:3338–41. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: Tests of an incentive motivational model. Biological Psychiatry. 2002;51:151–63. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD. Enhancement of continuous performance task reaction-time by smoking in nondeprived smokers. Psychopharmacology. 1992;108:437–42. doi: 10.1007/BF02247417. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, Macklin ML, Fischman AJ, Pitman RK. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Research-Neuroimaging. 1999;91:1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Rees G, Lavie N. What can functional imaging reveal about the role of attention in visual awareness? Neuropsychologia. 2001;39:1343–53. doi: 10.1016/s0028-3932(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette-smoking decreases cerebral blood-flow suggesting increased risk for stroke. Jama-Journal of the American Medical Association. 1983;250:2796–800. [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. Journal of Neuroscience. 1994;14:5437–52. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning A, Hernadi I. The neurophysiology of taste and olfaction in primates, and umami flavor. Annals of the New York Academy of Sciences. 1998;855:426–37. doi: 10.1111/j.1749-6632.1998.tb10602.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET Studies of the influences of nicotine on neural systems in cigarette smokers. American Journal of Psychiatry. 2003;160:323–33. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Dupont RM, Grant I, Lehr PP, Lamoureux G, Halpern S, Yeung DW, San Diego HIV Neurobehavioral Research Center Reduction in cortical IMP-SPET tracer uptake with recent cigarette consumption in a young group of healthy males. European Journal of Nuclear Medicine. 1997;24:422–7. doi: 10.1007/BF00881815. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Carr LA, Garner AC. Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. Journal of Neurochemistry. 1987;49:1449–54. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Caulfield D, King L, Goode A. Moving out of the laboratory: does nicotine improve everyday attention? Behavioural Pharmacology. 2000;11:621–9. doi: 10.1097/00008877-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha 4 nicotinic receptor subunit knockout mice. British Journal of Pharmacology. 2001;132:1650–6. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Takano Y, Kohjimoto Y, Honda K, Kamiya HO. Enhancement of [3H]dopamine release and its [3H]metabolites in rat striatum by nicotinic drugs. Brain Research. 1982;242:99–106. doi: 10.1016/0006-8993(82)90499-1. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvalahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. American Journal of Psychiatry. 2000a;157:632–4. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Salokangas RKR, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvalahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. American Journal of Psychiatry. 2000b;157:632–4. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, Svensson TH. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–83. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology (Berl) 1995;120:289–95. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relay functions. Vision: from Neurons to Cognition. 2001;134:51–69. doi: 10.1016/s0079-6123(01)34005-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Elash C, Kassel JD. Nicotine withdrawal in chippers and regular smokers – subjective and cognitive effects. Health Psychology. 1995;14:301–9. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Lowe AS, Williams SCR. Imaging localised dynamic changes in the nucleus accumbens following nicotine withdrawal in rats. Neuroimage. 2004;22:847–54. doi: 10.1016/j.neuroimage.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR, Pauly JR. Behavioural and biochemical adaptations to nicotine in rats: influence of MK801, an NMDA receptor antagonist. Psychopharmacology (Berl) 1997;134:121–30. doi: 10.1007/s002130050433. [DOI] [PubMed] [Google Scholar]
- Sihver W, Langstrom B, Nordberg A. Ligands for in vivo imaging of nicotinic receptor subtypes in Alzheimer brain. Acta Neurologica Scandinavica. 2000;102:27–33. doi: 10.1034/j.1600-0404.2000.00304.x. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 2002;357:1739–52. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroscience – storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Current Opinion in Neurobiology. 2003;13:663–70. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–84. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003a;28:765–72. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003b;28:765–72. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stein E, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao S, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: A functional MRI study. American Journal of Psychiatry. 1998;155:1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochemical Research. 2001;26:609–17. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Terborg C, Birkner T, Schack B, Witte OW. Acute effects of cigarette smoking on cerebral oxygenation and hemodynamics: a combined study with near-infrared spectroscopy and transcranial Doppler sonography. Journal of the Neurological Sciences. 2002;205:71–5. doi: 10.1016/s0022-510x(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Wilby G, Stough C. The effects of transdermal nicotine on inspection time. Human Psychopharmacology. 2002;17:157–61. doi: 10.1002/hup.377. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF. Comparative effects of methamphetamine and nicotine on the striatal [C-11]raclopride binding in unanesthetized monkeys. Synapse. 2002;45:207–12. doi: 10.1002/syn.10102. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Coulon C, Ottaviani M, Syrota A. Long-lasting occupancy of central nicotinic acetylcholine receptors after smoking: a PET study in monkeys. Journal of Neurochemistry. 2003;84:105–11. doi: 10.1046/j.1471-4159.2003.01502.x. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Guenther I, Coulon C, Hinnen F, Fuseau C, Ottaviani M, Crouzel C. Characterization of the nicotinic ligand 2-[F-18]fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine in vivo. Life Sciences. 1998;64:L93–7. doi: 10.1016/s0024-3205(98)00573-6. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Guenther I, Fuseau C, Coulon C, Ottaviani M, Crouzel C. Imaging central nicotinic acetylcholine receptors in baboons with [F-18]fluoro-A-85380. Journal of Nuclear Medicine. 1999;40:1374–80. [PubMed] [Google Scholar]
- Villemagne V, Horti A, Scheffel U, Ravert H, Finley P, Clough DJ, London E, Wagner H, Dannals RF. Imaging nicotinic acetylcholine receptors with fluorine-18-FPH, an epibatidine analog. Journal of Nuclear Medicine. 1997;38:1737–41. [PubMed] [Google Scholar]
- Westfall TC, Grant H, Perry H. Release of dopamine and 5-hydroxytryptamine from rat striatal slices following activation of nicotinic cholinergic receptors. General Pharmacology. 1983;14:321–5. doi: 10.1016/0306-3623(83)90037-x. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Natural Neuroscience. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nishiyama Y, Monden T, Satoh K, Ohkawa M. A study of the acute effect of smoking on cerebral blood flow using 99mTc-ECD SPET. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30:612–4. doi: 10.1007/s00259-003-1119-z. [DOI] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochemical Pharmacology. 1995;50:2001–8. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation. Pharmacology Biochemistry and Behavior. 1997;57:915–21. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Facilitatory modulation of mesolimbic dopamine neuronal-activity by a Mu-opioid agonist and nicotine as examined with in-vivo microdialysis. Brain Research. 1993;624:277–80. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tian JY, Svensson AL, Gong ZH, Meyerson B, Nordberg A. Chronic treatments with tacrine and (−)-nicotine induce different changes of nicotinic and muscarinic acetylcholine receptors in the brain of aged rat. Journal of Neural Transmission. 2002;109:377–92. doi: 10.1007/s007020200030. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biological Psychiatry. 2001;49:906–13. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]


