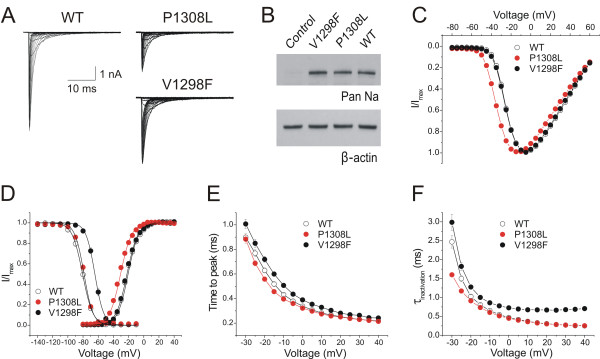
Figure 2.
P1308L and V1298F exhibit different effects on voltage-dependence of activation and fast inactivation. A, Representative families of traces of Na+ currents (INa) from voltage-clamped HEK293 cells stably expressing wild-type (WT), P1308L, or V1298F NaV1.7R channels. Cells were held at -100 mV, and Na+ currents were elicited by step depolarizations from -80 to +60 mV in 5 mV increments every 5 seconds. B. Western blot analysis of NaV1.7R WT and mutant channels in transfected HEK 293 cells. The loading variation was eliminated by normalizing the intensities of sodium channels with the intensities of β-actin of corresponding lanes. No statistic difference was observed between WT and mutant channels. C, Normalized peak current-voltage relationship for WT (n = 29), P1308L (n = 25), and V1298F (n = 26) NaV1.7R channels. D, Comparison of the voltage-dependent activation and steady-state fast inactivation of WT, P1308L, and V1298F channels. A hyperpolarizing shift (-9.6 mV) of activation was observed in the P1308L mutant channel, while the V1298F mutant channel showed a depolarizing shift (+16.1 mV) of steady-state fast-inactivation. E, Activation kinetics (measured as time-to-peak) of P1308L (n = 25) were faster at -20 mV and -15 mV, compared to WT channels (n = 29), whereas V1298F channels (n = 26) showed slower activation kinetics from -20 to +40 mV. F, Open-state fast-inactivation kinetics were measured by single-exponential fitting of the decay phases of INa as shown in (A). When compared with wild-type channels (n = 25), V1298F mutant channels (n = 26) significantly slowed the inactivation kinetics from -25 to +40 mV, whereas P1308L mutation (n = 19) showed faster inactivation kinetics at -30 and -25 mV.