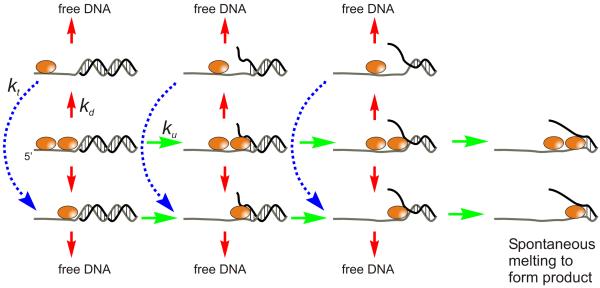
Figure 2.
Model for functional cooperativity for Dda helicase. Two Dda molecules are shown bound to the 14T16bp substrate (14 nt overhang and 16 bp of duplex DNA). Upon addition of ATP and Mg+2, DNA unwinding occurs according to rate constant ku (green arrows). Three kinetic steps are shown for unwinding of the 16 bp substrate (11). Either enzyme molecule can dissociate from the DNA substrate according to rate constant kd (red arrows). If the leading enzyme molecule dissociates, then the trailing molecule can translocate to the ss/ds junction according to rate constant kt. The blue dotted lines and arrows indicate two kinetic steps that are required for the trailing enzyme to move to the ss/ds DNA junction. The final base pairs can melt spontaneously giving rise to ssDNA product.