Abstract
Tissue morphogenesis during development is regulated by growth factors and cytokines, and is characterized by constant remodeling of extracellular matrix (ECM) in response to signaling molecules, for example, growth factors, cytokines, and so forth. Proteoglycans that bind growth factors are potential regulators of tissue morphogenesis during embryonic development. In this study, we showed that transgenic mice overexpressing biglycan under the keratocan promoter exhibited exposure keratitis and premature eye opening from noninfectious eyelid ulceration due to perturbation of eyelid muscle formation and the failure of meibomian gland formation. In addition, in vitro analysis revealed that biglycan binds to TGF-α, thus interrupting EGFR signaling pathways essential for mesenchymal cell migration induced by eyelid epithelium. The defects of TGF-α signaling by excess biglycan were further augmented by the interruption of the autocrine or paracrine loop of the EGFR signaling pathway of HB-EGF expression elicited by TGF-α. These results are consistent with the notion that under physiological conditions, biglycan secreted by mesenchymal cells serves as a regulatory molecule for the formation of a TGF-α gradient serving as a morphogen of eyelid morphogenesis.
Keywords: Biglycan, Eyelid morphogenesis, TGF-α, Muscle development, Keratocan promoter, Transgenic mouse
Introduction
In mammals, eyelid morphogenesis is characterized by the closure and fusion of the eyelid during development, which involves two major cellular events: the differentiation of ocular surface ectoderm to corneal, conjunctival, eyelid epidermal and glandular (lacrimal and meibomian) epithelia, and the migration and differentiation of periocular mesenchymal cells for the formation of eyelid stroma (Kao and Liu, 2003). Mouse eyelid formation begins at embryonic day 12 (E12), with folds of surface ectoderm adjacent to the developing eye. As it grows, the eyelid fold extends over the developing cornea, and periocular mesenchymal cells invade the eyelid stroma. The closure of the eyelid happens between E15.5 and E16.5, when the tips of the superior and inferior eyelid epidermis elongate toward the center of the eye and eventually cover the corneal surface (Findlater et al., 1993; Harris and Juriloff, 1986). Following epithelial fusion, the eyelid stroma grows by continued invasion and proliferation of mesenchymal cells, and a fusion line is formed between the superior and inferior eyelid epithelium to close the eye; thus, completing the formation of the eyelid protective barrier over the cornea. The mouse eyelids remain closed until 12–14 days postnatal. This process resembles the epithelial sheet migration of dorsal closure during Drosophila development (Glise and Noselli, 1997; Zhang et al., 2003), closure of choroid fissure of optic cup of vertebrates (Kurita et al., 2004; Macdonald and Wilson, 1996), and closure of fusion of chorioamniotic folds over platypus embryos (Hughes and Hall, 1998) and those of birds (Gilbert, 2003).
The eyelid serves as a protective barrier for the formation of the conjunctival sac and the development of the cornea and lens (Findlater et al., 1993; Harris and Juriloff, 1986). In normal mouse eye development, the commencement of eyelid formation coincides with the rapid growth of the eyeball beginning at E13.5. The formation of lid muscles, that is, orbicularis oculi, levator palpebral, and tarsal muscles, is essential for eyelid functions, which provide the adequate strength required to withstand the pressure exerted by the rapid growth of the eye. In addition, the tarsal plate at the end of the tarsal muscles provides the scaffold for the formation of meibomian glands that derive from epithelium at the tip of eyelids (Jester et al., 1989). The meibomian gland is a lipid-secreting gland that plays a pivotal role for the maintenance of tear film function. For example, meibomian gland dysfunction resulting from ectodermal dysplasia in human manifested severe exposure keratitis (Bron and Tiffany, 1998).
As in many other tissues, eyelid morphogenesis is also governed by bidirectional mesenchyme–epithelium interactions via growth factors, cytokines, and extracellular matrix (ECM) components during embryonic development. This is best illustrated by the eye open at birth phenotype (EOB) due to impaired epithelial cell migration in mouse strains in which the genes mediating signaling pathways of EGF, EGF receptors, and TGF-β superfamily members are ablated, for example, TGF-α, EGFR, cJun, MEKK 1, activin βB, and so forth (Li et al., 2003; Luetteke et al., 1993; Sibilia and Wagner, 1995; Vassalli et al., 1994; Zenz et al., 2003; Zhang et al., 2003). Thus, it is likely that genetic perturbations that impair epithelium migration can potentially cause eyelid malformation. However, it remains unknown whether mutation of these genes may impair the functions of periocular mesenchymal cells essential for eyelid morphogenesis.
Interestingly, failure in eyelid closure caused by spontaneous autosomal recessive mutations has been reported in several mouse strains, such as eye-open-at-birth (eob), lidgap-Gates (lgGa), open-eyes (oe), and gaping lids (gp) (Fujii et al., 1995; Juriloff et al., 1996; Stein et al., 1967; Teramoto et al., 1988). However, in each strain, the mutant gene responsible for EOB has not been identified (Juriloff et al., 1996, 2000; Kelton and Rauch, 1968). The obvious implication is that other molecular and cellular mechanism(s) besides epithelium migration may also be involved in eyelid morphogenesis. One of the many possibilities is a role for extracellular matrix components in eyelid morphogenesis, for example, proteoglycans, collagens, fibronectin, or laminin.
Proteoglycans belonging to the small leucine-rich repeat proteoglycan (SLRP) family, for example, decorin, biglycan, and fibromodulin, have been implicated as important molecules that bind and modulate the activities of growth factors and cytokines via their glycosaminoglycan moieties and core proteins (Kao and Liu, 2002; Iozzo, 1999; Reed and Iozzo, 2002). Biglycan contains two side chains of chondroitin sulfate/dermatan sulfate (CS/DS) glycosaminoglycans and is widely expressed by fibroblasts of interstitial connective tissues, mesothelial cells, and epithelial cells (Bianco et al., 1990; Dobra et al., 2000; Funderburgh et al., 1998). Like other members of the SLRP family, biglycan serves as a component of ECM and binds to a variety of other proteins, including growth factors such as TGF-β, TNF-α, and WISP-1 (Desnoyers et al., 2001; Hausser et al., 1994; Hildebrand et al., 1994); extracellular matrix proteins such as collagens I and V, fibronectin (Bidanset et al., 1992; Schmidt et al., 1987; Schonherr et al., 1995; Wiberg et al., 2003), and α-amyloid (Snow et al., 1995), apo E and apo B, α-dystroglycan, and phospholipase A2 type II (Bowe et al., 2000; Olin et al., 2001; Sartipy et al., 1998), and serum proteins such as heparin cofactor II (Fukushima et al., 1993; Hildebrand et al., 1994; Whinna et al., 1993). These various binding activities may account for the ability of biglycan to exert diverse functions in many tissues. For example, it has been suggested that biglycan may serve as a neurotrophic factor for the survival of neocortical neurons and participates in the regulation of neuronal growth, differentiation, and repair (Junghans et al., 1995; Koops et al., 1996). In addition, injection of biglycan into the brain apparently promoted the facilitation of learning (Huston et al., 2000). A recent study of rat neurons and C6 glioma cells revealed that a population of biglycan targets to the nucleus and may be involved in the regulation of neuronal cell division (Liang et al., 1997). Even though there is no conclusive evidence available, it is very likely that biglycan may also bind other growth factors, for example, TGF-α, activin βB, and so forth. Thus, biglycan is ideally situated to play a critical role in modulating the functions of growth factors in situ, and it is, therefore, conceivable that biglycan may play an important role in morphogenesis and tissue homeostasis.
We have created transgenic Kera-Bgn mouse lines using a 3.2-kb keratocan promoter (Liu et al., 2000), in which migrating periocular mesenchymal cells overexpress biglycan during embryonic development. The Kera-Bgn transgenic mice exhibited noninfectious eyelid ulceration causing premature eye opening and severe exposure keratitis. In this study, we present evidence illustrating that excess biglycan perturbs the formation of eyelid muscles and the migration of periocular mesenchymal cells by sequestering TGF-α secreted by eyelid epithelium during eyelid morphogenesis of mouse embryonic development.
Materials and methods
Generation of Kera-Bgn transgenic mice
The coding region of mouse biglycan cDNA was generated by RT-PCR using total RNA isolated from adult mouse corneas. The first strand cDNA was synthesized by AMV-reverse-transcriptase with random primers and was used to prepare the full-length double-stranded cDNA with a pair of HindIII linker primers (5′-mBgn: 5′-cccaagcttccaccatgtgtcccctgtggc-3′ and 3′-mBgn: 5′-cccaagcttggcaaccactgcctctac-3′). The double-stranded cDNA was cloned into the HindIII site of the mouse Kera promoter cassette Kerapr3.2-saBGHpA (Liu et al., 2000). The fidelity of the biglycan cDNA was verified by sequence analysis. The Kera3.2-Bgn/BGHpA minigene (5.2 kb) was isolated by NotI and KpnI digestion and microinjected into mouse-fertilized eggs by the transgenic core facility of the Cincinnati Children Hospital Research Foundation. Transgenic founders were identified by PCR of tail DNA using a primer set [Kera9 (+): 5′-ctaacaccagccacaggact-3′ and BGHpA (−): 5′-actagaaggcacagtcgagg-3′] flanking the biglycan cDNA.
All animal procedures were approved by the University of Cincinnati Animal Care and Use Committee. Timed mating procedure was used for obtaining mouse embryos at various developmental stages. Wild-type female mice were mated with a Kera-Bgn male at 9:00 p.m. and the next morning at 9:00 a.m. the females were examined for a coital plug, and 3:00 p.m. in the afternoon was defined as E0.5 of gestation when the evidence of copulation was detected. Cesarean-derived embryos at various developmental stages (E12.5 to 18.5) were genotyped by polymerase chain reaction with limb or tail DNA.
Northern hybridization
Total RNA was isolated from the corneas of the embryonic day 18.5 (E18.5) transgenic and wild-type littermates using TRI-reagent® (Molecular Research Center, Cincinnati, OH) (Liu et al., 1994), and 10 μg of each total RNA was subjected to Northern hybridization with 32P-labeled cDNA probes of mouse biglycan and glyceralde-hyde-3-phosphate dehydrogenase (GAPDH) as described previously (Liu et al., 1998).
Histology and immunohistochemistry
The embryos and adult eye tissues were fixed with 4.0% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at 4°C for 24 h, dehydrated, embedded in paraffin, and processed for histological examination. Histology of the eyes of the embryos was examined by light microscopy after hematoxylin and eosin and PAS staining.
For routine immunostaining, 5-μm paraffin sections were deparaffinized with xylene and rehydrated with graded ethanol. The sections were stained as described below and immune reactivity was visualized by Vecta stain ABC kit (Vector Laboratories), indirect alkaline phosphatase, or indirect immunofluorescence methods (Vector Laboratories and Molecular Probes). Primary antibodies used in this study were against: EGFR, pEGFR (Tyr1173), c-Jun, and pc-Jun (Santa Cruz, Santa Cruz, CA); TGF-α (Labvision); α-smooth muscle actin (Sigma); and biglycan (Kresse et al., 2001). The nuclei were counterstained with methyl green for immunohistochemistry using alkaline phosphatase conjugate, and propidium iodide (PI) or DAPI (Sigma) for immunofluorescence.
Scanning electron microscopy
The mouse embryos were fixed in 2.5% glutaraldehyde and 1% tannic acid prepared with 0.1 M phosphate buffer, pH 7.4. After postfixation with 1% osmic acid, the specimens were dehydrated with routine ethanol series. Following treatment with isoamyl acetate, the specimens were dried by the critical point method and then coated by platinum in an ion sputtering device. The prepared samples were examined with a scanning electron microscope (H-2000, Hitachi) (Zhang et al., 2003).
In situ hybridization
The plasmids of mouse biglycan and TGF-α cDNA cloned in pBluescript SK+ vector (Stratagene, CA) were linearized by EcoRI and SalI digestions, and T-7 and T-3 RNA polymerases were used to synthesize digoxigenin-conjugated antisense and sense riboprobes, respectively (Roche). Five-micrometer-thick paraffin sections were deparaffinized and processed for in situ hybridization with riboprobes of mouse biglycan and TGF-α, as previously reported (Kao et al., 1996; Saika et al., 2000, 2001). The sections were dehydrated, mounted, and observed under light microscopy after methyl green counterstaining.
Cell migration study
Isolation of eyelid epithelium by impression cytology
Filter membranes (2 mm in diameter) were cut from a Durapore® filter (pore size 0.45 μm; Millipore). To isolate epithelium, the membranes were pressed on top of the eyelid and head skin and immediately removed from the tissues. The membranes were then used in cell migration assays as described below (Hayashi et al., 2004).
Preparation of mesenchymal cell culture
The eyelid epithelium of wild-type E13.5 embryos was removed by impression cytology, and the remaining eyelid tissue was dissected under a stereomicroscope. The E13.5 embryo eyelid stromal tissues were washed in PBS three times and minced. Tissues were then cultured at 37°C in DMEM supplemented with 10% fetal calf serum in an incubator equilibrated with 95% air and 5% CO2 for 3 days. Cells from explant cultures were harvested with trypsin (1 mg/ml) and EDTA (5 mM) treatment, and subcultured on p100 cell culture dishes (Corning, NY) for three more days. Fresh medium was added to the culture overnight, prior to harvesting the cells for migration study. The culture condition used was unfavorable for the growth of epithelial cells, thus epithelial cells were absent in the culture as judged by a phase contrast microscope.
Cell migration assay
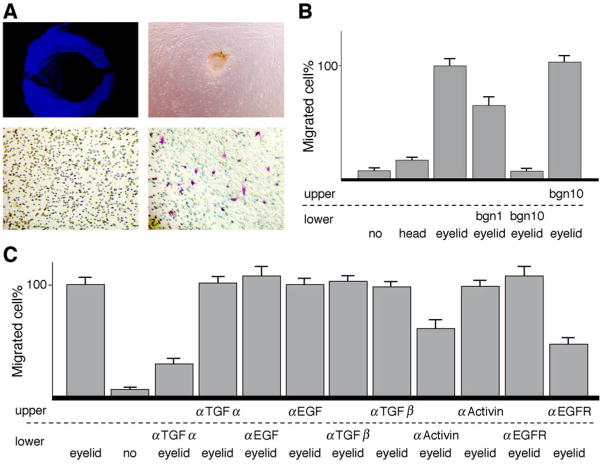
To measure cell migration, E13.5 eyelid and head epithelial tissues were prepared using impression cytology and were put on the bottom of the well containing 600 μl of serum-free DMEM (Gibco) of a 24-well plate. Cultured mesenchymal cells were harvested by treatment with trypsin-EDTA and washed two times with serum-free DMEM medium. Cells were suspended at 5 × 105 cells/ml in serum-free DMEM. One hundred microliters of cell suspension was seeded in each Transwells® (Costar) that was placed into a 24-well plate containing epithelium. Blocking antibody (2 μg/ml) and bovine tendon biglycan (1 or 10 μg/ml) were included in the culture medium of upper (Transwells®) or lower chambers (24-well plate), and incubated at 37°C for 4 h. Cotton swabs were used to remove cells from the upper surface of the Transwells®. The hematoxylin-stained migratory cells at the undersurface of Transwells® were counted with an inverted microscope. Each experimental condition was tested in quadruplicate in individual experiments, and the number of migratory cells found on the undersurface of Transwell® membranes of control experiments consisting of wild-type mesenchymal cells in the upper chamber and wild-type eyelid epithelial tissues in the lower chamber was defined as 100% migration index. Each experiment was repeated four times and statistical analyses were performed using unpaired t test.
Immune precipitation and Western blot
Pregnant female mice were sacrificed by CO2 asphyxiation on E13.5. Fetuses were removed from the uterus and dissected in PBS, and the limbs were removed for genotype identification by PCR. Biglycan extracts were prepared with an alkaline extraction method modified by Bowe et al. (2000) and Casar et al. (2004). Briefly, 10 eyelid tissues were homogenized in 50 mM Tris–NaOH, pH 12.0, containing 250 U/ml of Benzonase nuclease (Novagen, WI) and protease inhibitor cocktail (Sigma, MO), and maintained under agitation over night at 4°C. Then homogenates were centrifuged 30 min at 15,000 × g in 4°C, and supernatants were adjusted to pH 8.0 with 1 M Tris–HCl pH 6.0 and added Na acetate pH 8.0 (final concentration 100 mM), and centrifuged 10 min at 15,000 × g at 4°C. The supernatants were incubated with or without 0.5 U/ml chondroitinase ABC at 37°C for 1 h. Protein content in specimens was measured by BCA protein assay kit (Pierce, IL). For SDS-PAGE, 5 μg of protein of each sample was subjected to Western blot analysis as described below.
To prepare specimens for immune precipitation and Western blot (IP-Western), eyelid tissues were lysed in buffer solutions containing 50 mM Tris–HCl (pH 7.4), 250 mM NaCl, 25 mM NaF, 5 mM EDTA, 1 mM PMSF, 0.1% Nonidet P-40 in the presence of a protease inhibitor cocktail (Sigma) at 4°C for 24 h. Tissue lysates were clarified by centrifugation at 14,000 rpm, 4°C for 10 min. To remove nonspecific binding of protein to protein G-Sepharose, lysates were incubated with protein G-Sepharose (Amersham Biosciences) for 1 h. Slurries were spun down, and an aliquot of the supernatant was incubated with rabbit anti-TGFα, anti-TGFβ, anti-EGF, anti-activinβB, anti-HB-EGF, or anti-EGFR antibodies at 1 μg/ml IgG (Santa Cruz) at 4°C overnight. The antigen–antibodies complexes were precipitated with protein G-Sepharose conjugate. Immunoprecipitated proteins were electrophoresed on SDS-PAGE and blotted onto polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Immunoblots were treated with chondroitinase ABC, blocked with 5% dry milk in TBST (0.1% Triton X-100, 0.15 M NaCl, 50 mM Tris–HCl, pH 7.4), and probed with anti-biglycan antibody. After incubation with goat anti-rabbit IgG HRP conjugate, immunoblots were developed by the enhanced chemiluminescence system (Amersham Biosciences). Supernatant of eyelid homogenate in 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 10 mM EDTA, 0.5% sodium deoxycholate, 1 mM PMSF, 1% Nonidet P-40, 0.1% SDS, protease inhibitor cocktail was subjected to Western blot analysis to identify growth factors, receptors, and c-Jun as previously described (Saika et al., 2000).
Confocal microscopy
Developing embryos were evaluated at days 15.0, 15.5, 17.0, and 17.5 of gestation. Samples were fixed in 4% paraformaldehyde in PBS, and developing eyes and surrounding lids removed and stained with the following combinations of probes: (1) FITC-conjugated mouse anti-α-smooth muscle actin (α-SMA) and rhodamine phalloidin, or (2) FITC-conjugated phalloidin and propidium iodide. Samples were then mounted on mylar bottom plates (25 μm thick) (Invitrogen) or further dissected to include just the developing lid tissues and mounted on glass slides with PBS/glycerol (1:1). Samples were viewed using a Leica SP2 confocal microscope with argon/krypton and helium/neon lasers providing excitation frequencies of 488, 514, 543, and 633 nm. Three-dimensional data sets were collected from each sample and reconstructed using the Leica three-dimensional software package including maximum intensity projections for identifying localization of actin and α-SMA.
Results
Phenotype of Kera-Bgn transgenic mice
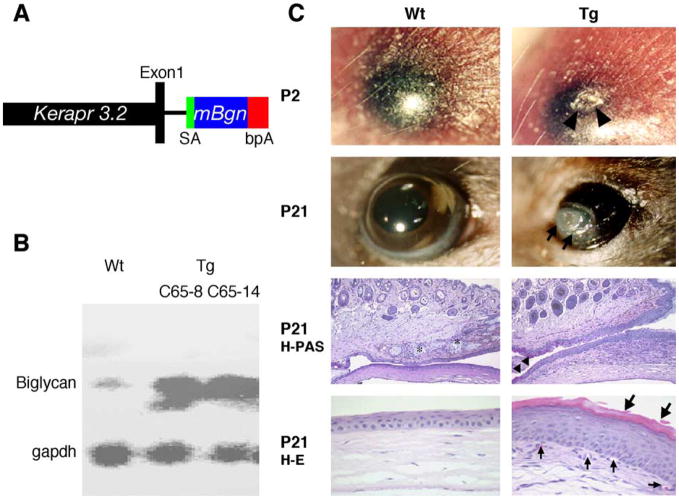
A 3.2-kb murine keratocan promoter was used to create transgenic mice that overexpress biglycan by migrating periocular mesenchymal cells during eyelid development (Liu et al., 2000). Two independent transgenic Kera-Bgn mouse lines (C65-8 and C65-14) were obtained via micro-injection of fertilized mouse eggs with a minigene containing a 3.2-kb keratocan promoter and a mouse biglycan cDNA (Fig. 1A). Due to the exposure keratitis observed in postnatal transgenic mice (Fig. 1C), embryonic corneas were used to determine the expression level of biglycan in corneas by Northern hybridization. As shown in Fig. 1B, at E18.5 the transgenic mice have a higher level of biglycan mRNA in the cornea than that of wild-type littermates. Both transgenic lines had the same phenotype of premature eye opening due to noninfectious ulceration at postnatal day 1 (P1) or P2 and suffered severe exposure keratitis as they grew older (Fig. 1C). Histology examinations revealed that the transgenic mice at P21 exhibited the lack of meibomian glands, malformation of the tarsal plate, epithelial hyperplasia with the formation of a cornified envelope and the presence of goblet cells on the peripheral corneal surface, and vascularization and invasion of inflammatory cells in the corneal stroma, suggesting conjunctivalization of the corneal surface.
Fig. 1.
Generation and phenotypes of Kera-Bgn transgenic mice. (A) Diagram of Kera-Bgn minigene, in which the mouse biglycan cDNA (mBgn) is flanked by the mouse keratocan promoter cassette Kerapr3.2-saBGHpA. (B) Northern blot analysis showing biglycan mRNA overexpression in the E18.5 corneas of two transgenic mouse lines. (C) Histology of Kera-Bgn mice showing premature eye open phenotype in Kera-Bgn mice due to noninfectious ulceration (arrowheads) at P2. The Kera-Bgn mice suffered severe exposure keratitis at P21 and are exemplified by the lack of meibomian glands (asterisk), the presence of goblet cells (arrowheads), and cornified epithelium (large arrows) at the corneal surface, and vascularized corneal stroma (small arrows). Wt, wild type; Tg, transgenic; bpA, bovine growth factor polyadenylation signal; H–E, hematoxylin and eosin staining; H-PAS, hematoxylin and periodic acid Schiff staining; SA, splice acceptor site.
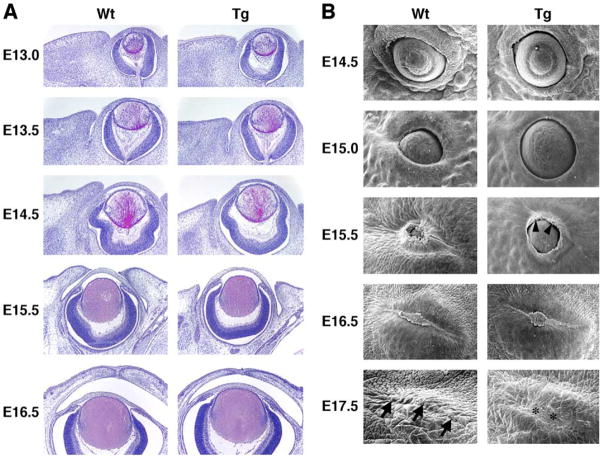
To examine whether the phenotype observed is a primary defect caused by excess biglycan, we checked the eyelid development of transgenic mouse strains by histology and scanning electron microscopy. In Kera-Bgn mice, eyelid malformation started at E13.5 (Fig. 2A). Kera-Bgn transgenic mouse eyelid closure was delayed by about half a day in comparison to wild-type littermates. After eyelid closure, the eyelid stroma was thinner and there were fewer stromal cells than in wild-type littermates. By scanning electron microscopy study, the elongation at the leading edge of the Kera-Bgn mice epithelium was irregular compared to the wild-type littermates. Ulceration of the eyelid fusion area was already apparent in E17.5 Kera-Bgn mice (Fig. 2B). During embryonic development, Kera-Bgn mice showed normal expression patterns of Krt12 (keratin 12 gene) by corneal epithelium and Krt1/Krt10 by eyelid epidermal epithelium similar to that of wild-type mice (data not shown). This observation is consistent with the notion that changes of corneal surface observed in postnatal transgenic mice are secondary to the pathogenesis of exposure keratitis.
Fig. 2.
Eyelid development in wild type and Kera-Bgn transgenic mice. (A) Horizontal (E13.0 and E13.5) and coronal (E14.5 to E16.5) sections of developing mouse eye (H–E stain). Eyelid stroma of Kera-Bgn (Tg) mice is thinner and has fewer cells compared to wild-type (Wt) littermates. In wild-type mice, eyelids fused between E15.5 and E16.5. In Kera-Bgn mice, the eyelid closure is delayed as compared to wild type. (B) Scanning electron microscopy of mouse eyelid development. Delay of eyelid closure was seen in Kera-Bgn (Tg) mice (E14.5 to E16.5). The elongation direction of the leading edge of the Kera-Bgn mouse epithelium (arrowheads) is irregular just before eyelid closure (E15.5). Ulceration occurs in the eyelid fusion area (*) of E17.5 Kera-Bgn mice, whereas tightly fused eyelids are seen in wild-type (Wt) mice (arrows).
Eyelid muscle development
As shown in Fig. 1C, the Kera-Bgn mice at P21 exhibited lack of the tarsal plate. To examine whether excess biglycan impairs the formation of eyelid muscles, for example, orbicularis oculi, levator palpebrae, and tarsal muscles, confocal microscopy using Phalloidin Rhodamine (F-actin) and anti-α-smooth muscle actin (α-SMA) antibodies was performed during the development of wild type and Kera-Bgn mice.
Wild-type mouse
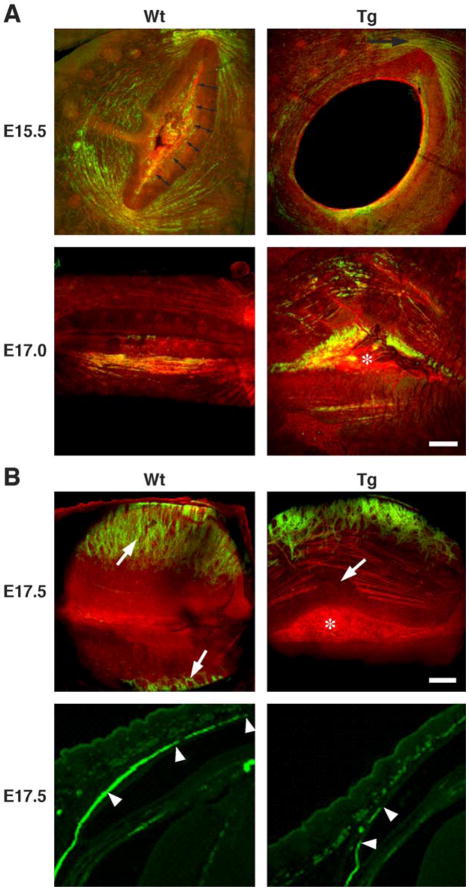
Early development of orbicularis oculi muscles (green) was detected at the lateral and medial margins of the lids by anti-α-SMA staining. By E15.5 (Fig. 3A, top-left), rapid development of the orbicularis oculi muscles was noted with muscle fibers extending from the lateral to medial insertions of the muscle to completely circumscribe the eyelid margins. Muscle development appeared to parallel lid closure and the formation of presumptive tarsal plate (double headed arrows) that excluded any developing muscles fibers. Closure of the lid margins at E17.0 was characterized by prominent development of the orbicularis oculi muscle with muscle fibers running almost vertically alongside of the presumptive tarsal plate (Fig. 3A, bottom-left, arrowheads). At the time of eyelid fusion, prominent muscle bundles from the orbicularis oculi ran directly along the presumptive tarsal plate, vertically from the lateral to the medial insertion. At E17.5, development of the levator palpebrae and tarsal muscles underlying the palpebral conjunctiva was detected and characterized by anti-α-SMA staining of developing muscles running vertically across the orbicularis oculi toward the developing tarsal plate (Fig. 3B, top-left, arrows and bottom left, arrowheads).
Fig. 3.
Development of eyelid orbicularis oculi, and levator palpebrae and tarsal muscles. (A) Confocal three-dimensional maximum intensity projections of the outer layers (epidermal side) of the developing orbicularis oculi from wild type (Wt) and Kera-Bgn (Tg) mice at E15.5 and E17.0. Lids are stained by anti-α-SMA staining (green) and phalloidin (red). Developing orbicularis oculi muscle shows continuous anti-α-SMA staining circumferentially around the eyelids and presumptive tarsal plate (double headed arrows) in wild type (Wt). At the same developmental stage, Kera-Bgn mice showed muscle development limited to the medial and lateral (top right panel, arrow) aspects of the eyelids. This developmental stage was approximately 12 h behind that detected for the wild-type (Wt) lid. The presumptive tarsal plate never appeared to become compacted, and the area is covered by remnant epidermal epithelium (asterisk). (B) Confocal three-dimensional maximum intensity projections of the outer layers (top panels, palpebral side) and immunofluorescent staining (bottom panels) of the developing eyelid from wild type (Wt) and Kera-Bgn (Tg) mice at E17.5. In wild-type mice, the tarsal muscle bundles run vertically toward the lid tip (top left panel, arrows; bottom left panel arrowheads). In Kera-Bgn (Tg) mice, the lid margins never fuse (top right panel, asterisk) with breaks in the developing orbicularis muscle fibers (top right panel, arrow). Development of the tarsal muscles is also delayed and fragmented (right bottom panel, arrowheads).
Kera-Bgn transgenic mouse
Initial development of the orbicularis oculi muscle at the medial and lateral margins of the eyelid was first detected at E15.5 with staining by anti-α-SMA (Fig. 3A, top-right). Staining of the developing muscle was noted to be at least 12 h behind that observed for wild-type littermates. α-SMA-stained muscle bundles failed to develop circumferentially around the developing eyelid margin (Fig. 3A, bottom-right). Additionally, the presumptive tarsal plate never appeared to become compacted and appeared much wider than that observed in lids from wild-type mice. It should be noted that muscle bundles never developed running parallel to the presumptive tarsal plate and that the margins of the developing lid mesenchyme never fused but remained separated by epithelium covering the surface of the eye (Fig. 3A, bottom-right, asterisk). Evaluation from the palpebral conjunctival side of the lid also showed prominent interruption in the developing orbicularis oculi muscle bundle (Fig. 3B, top-right, arrow), and the development of the levator palpebrae and tarsal muscles was delayed and showed more irregular development of muscle fibers (Fig. 3B, top-right; bottom-right, arrowheads) compared to the wild-type lid (Fig. 3B, top left, arrows; bottom left, arrowheads).
Biglycan expression during eyelid development of Kera-Bgn mice
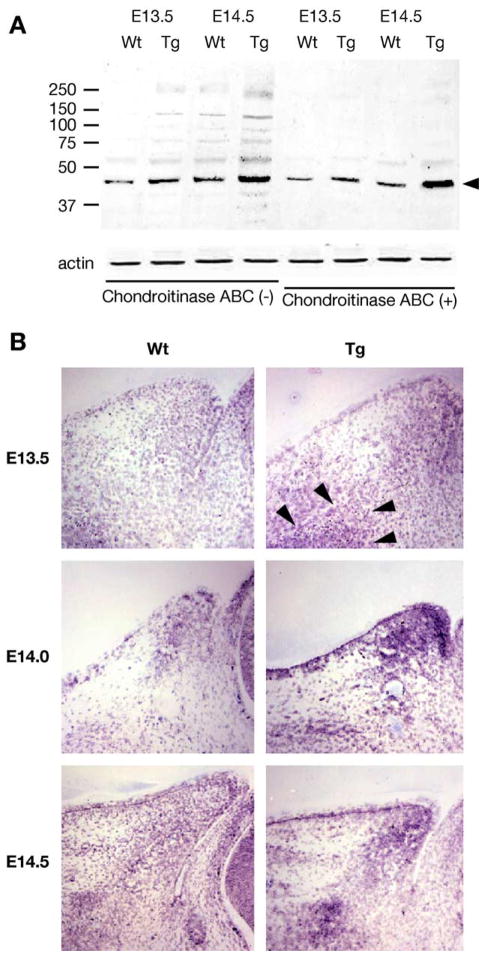
Western blot analysis was used to determine whether there was more biglycan present in the eyelid of transgenic mice. Biglycan extracted from wild type and transgenic mice was treated with or without chondroitinase ABC prior to PAGE-SDS. As shown in Fig. 4A, more biglycan was recovered from tissue extracts prepared from transgenic mice than the wild type at E13.5 and E14.5. The biglycan core protein is detected in molecular size ranges from about 46 kDa to larger than 250 kDa. The high molecular weight bands are sensitive to chondroitinase ABC digestion, indicating the biglycan exists as a CS/DS-proteoglycan having various degrees of posttranslational modifications.
Fig. 4.
Expression of biglycan determined by Western blot and in situ hybridization. (A) Protein extracts of eyelids from E13.5 and E14.5 wild type (Wt) and Kera-Bgn (Tg) mice were treated with and without chondroitinase ABC (0.5 unit/ml) prior to Western blot analysis with rabbit anti-biglycan antibodies. Actin determined by Western analysis was used to normalize the amounts of proteins applied to the gels. The biglycan existed as a heterogeneous CS/DS proteoglycan with molecular weights ranging from 46 kDa to greater than 250 kDa. The high molecular proteins reacted by the antibodies were digested by the enzyme and migrate as the 46 kDa biglycan core protein. (B) In situ hybridization reveals that biglycan mRNA was expressed by migrating mesenchymal cells of eyelid from wild-type (Wt) mice. In Kera-Bgn (Tg) mice, migrating mesenchymal cells (arrowheads) expressing biglycan accumulate under the epithelium adjacent to the palpebral side of conjunctiva at the tip of the lid from E13.5 through E14.5, with an interrupted pattern throughout the eyelid stroma as compared to wild-type (Wt) mice at E14.5.
In situ hybridization reveals that in wild-type mice, mesenchymal cells expressing biglycan first appeared at the base of the nasal eyelid fold at E13.5 and invaded into the eyelid stroma at E14.0 and spread along a path from the base to the tip of eyelid stroma at E14.5 (Fig. 4B, left panels). In Kera-Bgn mice, the migrating mesenchymal cells expressing biglycan also appeared at the base of the lid (Fig. 4B, top right, arrow heads). Interestingly, in contrast to wild-type mice, in Kera-Bgn mice stronger hybridization signals were detected, suggesting more mesenchymal cells or the mesenchymal cells expressing a higher level of biglycan mRNA appeared concentrated at the eyelid tip at E14.0 (Fig. 4B, middle-right), and at the tip and base of the eyelid at E14.5 (Fig. 4B, bottom right). These observations of the distribution of biglycan-expressing cells in transgenic mice implicate that the initial wave of mesenchymal cell migration is not compromised, but the continued mesenchymal cell migration is perturbed by excess biglycan expression during development.
As shown in Fig. 2, the eyelids of Kera-Bgn mice had thinner stroma and fewer cells as compared to wild-type mice, we investigated the possibility that this morphological abnormality in the presence of excess biglycan could derive from decreased cell proliferation, increased apoptosis, or impaired mesenchymal cell migration. To examine these three hypotheses, we first determined cell proliferation activities by BrdU incorporation and apoptosis by TUNEL assay, and found there was no difference in BrdU incorporation and cell apoptosis between wild type and Kera-Bgn mice (data not shown). Thus, the eyelid thinning and decrease of eyelid stromal cells may be due to the perturbation of mesenchymal cell migration rather than altered cell proliferation and apoptosis in transgenic mice.
Cell migration study
To examine the possibility that excess biglycan might perturb cell migration in developing eyelids, a two-chamber culture system was designed in which eyelid epithelium obtained by impression cytology was placed in the lower chamber, and eyelid mesenchymal cells were seeded on a Transwell® membrane in the upper chamber. The epithelium and mesenchymal cells were co-cultured at 37°C in a CO2 incubator for 4 h. The number of mesenchymal cells that migrated toward the epithelium and across the transmembrane pores was determined as described in Materials and methods. When eyelid epithelial tissues were used, many eyelid mesenchymal cells migrated to the opposite side of the Transwell® membrane as shown in Fig. 5A. In the first series of experiments, the eyelid epithelium was prepared from mouse embryos at different gestations E13.5, 14.5, and 15.5 by impression cytology. It was found that the eyelid epithelium from E13.5 embryos produced the highest chemoattractive activity on mesenchymal cells (data not shown). E13.5 embryos were then used in all the following experiments.
Fig. 5.
Mesenchymal cell migration induced by eyelid epithelium. (A) DAPI staining of impression cytology taken from E13.5 wild-type eyelid epithelium (top left). Primary eyelid stromal cells derived from explant culture of E13.5 eyelid at day 3 (top right). Cultured eyelid stromal mesenchymal cells migrate across the Transwell® membrane when eyelid epithelium is placed in the lower chamber (bottom right). Few mesenchymal cells migrate across the membrane in the absence of eyelid epithelium (bottom left). (B) Bovine biglycan (1 and 10 μg/ml) added to the lower chamber suppresses eyelid mesenchymal cell migration, whereas biglycan added to the upper chamber does not perturb cell migration. (C) Addition of neutralizing antibodies (1 μg/ml) against TGF-α or activin βB to the lower chamber and EGF receptor antibodies to the upper chamber significantly suppresses mesenchymal cell migration, but not anti-TGF-α, anti-TGF-βs, anti-EGF, and anti-activin βB in the upper or EGFR in the lower chamber.
To examine whether biglycan might impair mesenchymal cell migration, biglycan (1 and 10 μg/ml) was included in the culture medium of either the lower or upper chamber. Fig. 5B demonstrates that biglycan added in the lower chamber containing eyelid epithelium effectively blocked the migration of mesenchymal cells from the upper chamber toward the epithelium placed in the lower chamber, whereas biglycan added in the upper chamber did not perturb mesenchymal cell migration. This observation is consistent with the notion that biglycan may bind to and abolish the activity of chemoattractive factor(s) secreted by the eyelid epithelium.
It has been demonstrated that the perturbation of EGFR and activin βB signaling pathways leads to the eye open phenotype in mouse strains. Thus, we tested the possibility that biglycan might bind to and sequester growth factors, for example, TGF-α, EGF, TGF-β, and activin βB, secreted by eyelid epithelium. Neutralizing antibodies to such growth factors were included in the culture medium. As shown in Fig. 5C, addition of anti-TGF-α antibodies, but not anti-EGF or anti-TGF-β antibodies, in the lower chamber strongly inhibits cell migration induced by E13.5 wild-type epithelium. Addition of anti-activin βB antibodies produced a moderate inhibition of cell migration. Neutralizing antibodies of TGF-α, TGF-β, EGF, and activin βB present in the upper chamber did not perturb mesenchymal cell migration. The presence of neutralizing antibodies against EGFR in the upper chamber inhibits eyelid stromal cell migration, suggesting that eyelid mesenchymal cell migration is mediated via the TGF-α/EGFR signaling pathway.
Detection of Bgn-TGF-α complex by immune precipitation–Western blot analysis
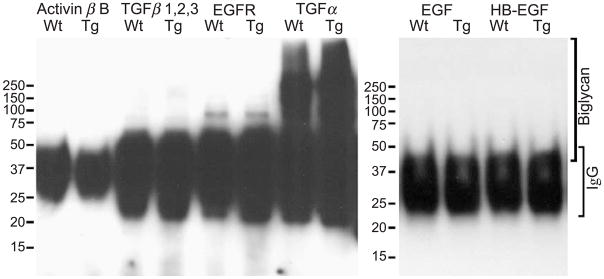
As mentioned above, excess biglycan may sequester TGF-α and abolish the TGF-α-induced periocular mesenchymal cell migration by disrupting the gradient of the growth factor in eyelid stroma morphogenesis during embryonic development of Kera-Bgn mice. To verify the existence of such a growth factor–biglycan complex in developing eyelids, proteins were extracted from eyelids of E13.5 mouse embryos and incubated with antibodies against TGF-α, TGF-β, activin βB, EGF, HB-EGF, and EGFR. The potential antigen–antibody complexes were precipitated by protein G-Sepharose conjugates and subjected to Western blot analysis with rabbit anti-biglycan antibodies. As shown in Fig. 6, only anti-TGF-α antibodies co-precipitated biglycan from eyelid extracts, but not anti-activin βB, TGF-β, EGFR, EGF, or HB-EGF antibodies. The results support the suggestion that biglycan does bind to TGF-α.
Fig. 6.
Binding of biglycan to TGF-α. E13.5 eyelid tissue extracts from wild type (Wt) and Kera-Bgn (Tg) mice were incubated with anti-TGF-α, TGF-β, activin βB, EGF, HB-EGF, or EGFR antibodies. The antigen–antibody complexes were precipitated with protein G-Sepharose conjugates and subjected to Western blot with anti-biglycan antibodies. Only use of the anti-TGF-α antibody co-precipitated TGF-α and biglycan. The reactive material at molecular weights between 30 and 50 kDa represents the IgG of the primary antibodies.
Tissue distribution of TGF-α, EGFR, phosphorylated EGFR, and AP1
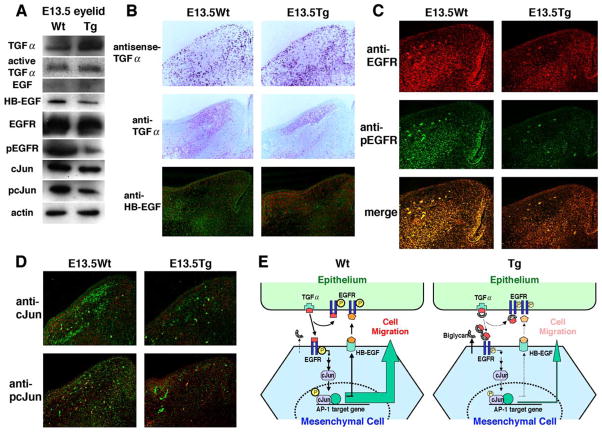
The results described above indicate that excess biglycan can sequester TGF-α and block the growth factor from serving as a chemoattractant for mesenchymal cell migration during eyelid morphogenesis. Thus, it is predictable that overexpression of biglycan by migrating periocular mesenchymal cells may alter the distribution of TGF-α in developing eyelid and consequently perturb the signaling pathways, for example, activation of EGFR and AP 1 transcription factors. To examine these possibilities, we examined expression levels and localizations of TGF-α, EGFR and phosphorylated EGFR (pEGFR), HB-EGF, and cJun and phosphorylated cJun (pcJun) in the developing eyelids of E13.5 Kera-Bgn and wild-type mice. Western blot analysis showed similar levels of active TGF-α and EGFR; an increase of total TGF-α; decreases of cJun and HB-EGF; and reduction of pEGFR in Kera-Bgn mice compared to wild-type mice (Fig. 7A). In situ hybridization revealed that TGF-α was highly expressed by epidermal epithelium of eyelid and migrating periocular mesenchymal cells of both Kera-Bgn and wild-type mice (Fig. 7B). However, immunohistochemistry with anti-TGF-α indicates that in transgenic mice the growth factor was unevenly distributed and more abundant in the upper one-third portion of the eyelid adjacent to epidermis, whereas in wild-type mice the growth factor was widely distributed and extended through more than two-thirds of the developing eyelid at E13.5. This pattern of TGF-α distribution is consistent with the overexpression of biglycan by mesenchymal cells of Kera-Bgn mice, as shown in Fig. 4. Furthermore, the expression of HB-EGF was suppressed (Fig. 7B) and the activation of EGFR (phosphorylation) was down-regulated in the eyelids of Kera-Bgn mice (Fig. 7C). As shown in Fig. 7D, excess biglycan caused a slight decrease of c-Jun protein, but strongly inhibited the activation of c-Jun as shown by a decrease of pcJun in the eyelids of Kera-Bgn mice.
Fig. 7.
Expression of TGF-α, EGF, and HB-EGF, and activation of EGFR and cJun during eyelid morphogenesis of Kera-Bgn mice. (A) Western blot analysis of TGF-α, EGF, HB-EGF, EGFR, pEGFR, cJun, and pcJun of eyelid extracts from E13.5 embryos shows downregulated expression of HB-EGF and cJun, and phosphorylation of EGFR and cJun in Kera-Bgn (Tg) mice. (B) In situ hybridization detects TGF-α mRNA in epithelium and migrating mesenchymal cells of wild-type (Wt) and Kera-Bgn (Tg) eyelid. Immunohistochemistry shows TGF-α distribution is limited to the upper one-third of eyelid stroma adjacent to the epithelium of Kera-Bgn (Tg) mice, but it is more evenly distributed along the stroma of wild-type (Wt) mice. The expression of HB-EGF (green) is also downregulated in the eyelids of Kera-Bgn mice (counter stained with propidium iodide, red). (C) EGFR (red) and pEGFR (green) are detected in migrating mesenchymal cells and epidermal epithelium of E13.5 wild-type (Wt) eyelid, whereas lower pEGFR positive signals are observed in Kera-Bgn (Tg) littermates. (D) cJun and pcJun expression (green) in migrating mesenchymal cells. The tissue sections are counterstained with propidium iodide (red). cJun is expressed at E13.5 by both wild-type (Wt) and Kera-Bgn (Tg) eyelid mesenchymal cells. Fewer cells express pcJun in Kera-Bgn (Tg) eyelid stroma compared to wild-type littermates. (E) Diagrams illustrating the EGFR signaling mediated by TGF-α during eyelid morphogenesis in wild-type (Wt) and Kera-Bgn (Tg) mice. The presence of excess biglycan in transgenic mice sequesters TGF-α and consequently perturbs the autocrine or paracrine loop of EGFR signaling pathways via HB-EGF and impairs mesenchymal cell migration.
Discussion
In the present studies, we have characterized the Kera-Bgn transgenic mice that exhibit premature eye opening and severe exposure keratitis characterized by epithelial hyperplasia and the formation of a cornified envelope at the corneal surface. These defects are secondary to the mal-development of eyelids with thin stroma, lack of tarsal plates and meibomian glands, and perturbed maturation of lid orbicularis oculi, and levator palpebrae and tarsal muscles. In contrast in wild-type mice, compaction of the presumptive tarsal plate directly coincided with the development of muscle fibers running parallel to the presumptive tarsal plate, presumably associated with the developing pars palpebralis portion of the orbicularis oculi muscle that inserts into the medial ligament. These findings taken together suggest that development of lid and associated lid muscles plays a critical role in lid closure and fusion. Furthermore, excessive biglycan expression appears to interfere with critical EGFR signal transduction events involving TGF-α.
Role of lid muscles in eyelid morphogenesis
Tension within the muscle exerted during development might provide mechanical forces that keep the lids closed during development. Additionally, it should be noted that the eye extends out beyond the surface of the lids early in development. As the muscles develop, contraction of the muscle would tend to pull the lids across the eye and help push the eye farther back into the orbit.
Data concerning the Kera-Bgn mice suggest that eyelid open at birth may be related to a major defect in the development of eyelid muscles. Failure of the normal development of this muscle might lead to a failure in the fusion of the lids due to the mal-approximation of the mesenchymal portion of the developing eyelids. As the eye grows, the failure of the muscles to align parallel to the tarsal plate might lead to structural failure as the growing eyeball pushes forward. At some point during development, the eyeball would then push open the lids resulting in the eyelid open at birth phenotype. The malformation of tarsal muscle in Kera-Bgn mice fails to provide a scaffold for the formation of meibomian glands. The lack of meibomian glands contributes to the severe exposure keratitis observed in Kera-Bgn mice resembling the pathogenesis of human diseases of meibomian glands dysfunction from ectodermal dysplasia (Bron and Tiffany, 1998).
However, the molecular and cellular mechanism(s) whereby excess biglycan impairs lid muscle formation remains unknown. During avian eye development, it has been suggested that eyelid muscles may be also derived from periocular mesenchymal cells that give rise to stromal cells of many ocular tissues, for example, cornea, iris, ciliary body, and eyelids (Noden, 1986). It remains unknown whether malformation of orbicularis oculi, levator palpebrae, and tarsal muscles of Kera-Bgn mice is due to impaired mesenchymal cell migration or perturbed differentiation of mesenchymal cells, or both. It has been demonstrated that biglycan binds to α-dystroglycan and that in muscle biglycan is detected at both synaptic and nonsynaptic regions. Furthermore, biglycan expression is upregulated in muscle from dystrophic mdx mice (Bowe et al., 2000). Thus, our observation of malformation of lid muscles in transgenic mice is consistent with the notion that upregulated biglycan expression may account for the pathogenesis of some muscular dystrophies. Further studies are needed to elucidate the molecular and cellular mechanism(s) of eyelid muscle development, and whether the mal-development of eyelid muscles is directly caused or is mediated by the perturbation of EGFR signaling pathways due to excess biglycan.
Migration of periocular mesenchymal cells during eyelid morphogenesis
Epithelium sheet migration induced by growth factors or cytokines plays a pivotal role in tissue morphogenesis during embryonic development. For example, TGF-β signaling is essential for dorsal closure in Drosophila (Glise and Noselli, 1997). Genetic alterations in many knockout and transgenic mouse strains that exhibit an eye open at birth phenotype are characterized by impaired epithelium sheet migration (Carroll et al., 1998; Li et al., 2003; Luetteke et al., 1993; Sibilia and Wagner, 1995; Vassalli et al., 1994; Zenz et al., 2003; Zhang et al., 2003). Many signal transduction pathways, including activin βB, TGF-α/EGFR, MEKK1, and c-Jun, are involved in eyelid closure. In addition, mutations of molecules mediating cell migration, for example, integrins, fibronectin, and so forth, are also exemplified by the eye open at birth phenotype. It has been demonstrated that mutation of these genes also causes defective cell migration of cultured epithelial cells in vitro.
In the present studies, our data demonstrate for the first time that TGF-α signaling also plays an important role in regulating the migration of periocular mesenchymal cells into the eyelid stroma during development. Mesenchymal cell migration also appears to be independent of TGF-β signal transduction, as shown in our previous studies of TGF-β knockout mice that do not exhibit mal-development of the eyelid (Saika et al., 2001). However, inhibition of cell migration by activin βB-neutralizing antibodies is consistent with the eye open at birth phenotype observed in activin βB knockout mice (Vassalli et al., 1994), suggesting that activin βB may also serve as a chemoattractant to periocular mesenchymal cells during eyelid morphogenesis.
Autocrine and paracrine loops of TGF-α signaling during eyelid morphogenesis
Interestingly, the present studies provide evidence that EGFR signaling pathways play a pivotal role in periocular mesenchymal cell migration. The disruption of the TGF-α gradient necessary for mesenchymal cell migration by excess biglycan is accompanied by diminished phosphorylation of EGFR and cJun and the suppression of HB-EGF (Fig. 7). Thus, excess biglycan perturbs the autocrine or paracrine loops of the EGFR signaling pathways required for normal lid morphogenesis (Fig. 7E). EGF receptor signaling is essential for eyelid morphogenesis as evidenced by spontaneous mutations or knockout mouse strains in which this signaling pathway is interrupted. For example, ablation and spontaneous mutation of TGF-α and ablation of EGFR elicit the eye open at birth phenotype (Luetteke et al., 1993; Mann et al., 1993; Reneker et al., 1995). The TGF-α signaling is probably mediated by MAP kinase pathways. Recently, it has been shown that conditional ablation of cJun in the epidermis leads to the eye open at birth phenotype. In vitro analysis using cultured epithelial cells revealed that the lack of these gene products is associated with impaired epithelial cell migration and interruption of the autocrine and paracrine loops of EGFR signaling pathways (Li et al., 2003; Zenz et al., 2003). It is of interest to note that ablation of MEKK 1 also elicits an eye open at birth phenotype. In vitro examination of cultured epithelial cells isolated from skin of mice lacking MEKK 1 kinase activities indicates that MEKK 1 mediates the activin βB signaling pathways (Zhang et al., 2003).
In summary in the present studies, we have demonstrated for the first time that excess biglycan perturbs eyelid muscle formation that can in part explain the eye open at birth phenotype of Kera-Bgn mice. Also, excess biglycan in the eyelid stroma of transgenic mice sequesters TGF-α and disrupts the formation of the TGF-α gradient that is necessary for guiding mesenchymal cell migration and consequently interrupts the EGFR signaling loops necessary for eyelid morphogenesis (Fig. 7E). Thus, it is intriguing to suggest that the ability of biglycan to bind TGF-α allows this CS/DS-proteoglycan to serve as a regulatory molecule for the formation of a TGF-α gradient in eyelid morphogenesis during normal embryonic development.
Acknowledgments
The studies were in part supported by NIH grants: EY 11845, EY 13755, EY 12486, EY 09368, EY13215, and EY07348; Research To Prevent Blindness (RPB); the Ohio Lions Eye Research Foundation; the Shriners of North America; the Canadian Institutes for Health Research; and the Japan Eye Bank Association. WWYK and JJJ are the recipients of the RPB Senior Scientific Investigator Award. The authors thank Dr. Ying Xia, University of Cincinnati, for her suggestions in designing the cell migration assay.
References
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148:801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- Carroll JM, Luetteke NC, Lee DC, Watt FM. Role of integrins in mouse eyelid development: studies in normal embryos and embryos in which there is a failure of eyelid fusion. Mech Dev. 1998;78:37–45. doi: 10.1016/s0925-4773(98)00145-2. [DOI] [PubMed] [Google Scholar]
- Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol. 2004;268:358–371. doi: 10.1016/j.ydbio.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- Dobra K, Andang M, Syrokou A, Karamanos NK, Hjerpe A. Differentiation of mesothelioma cells is influenced by the expression of proteoglycans. Exp Cell Res. 2000;258:12–22. doi: 10.1006/excr.2000.4915. [DOI] [PubMed] [Google Scholar]
- Findlater GS, McDougall RD, Kaufman MH. Eyelid development, fusion and subsequent reopening in the mouse. J Anat. 1993;183 (Pt. 1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Hatakenaka N, Kaneda M, Teramoto S. Morphogenetic study of the eyelids in NC-eob mice fetuses with an open-eyelid malformation at birth. Lab Anim Sci. 1995;45:176–180. [PubMed] [Google Scholar]
- Fukushima D, Butzow R, Hildebrand A, Ruoslahti E. Localization of transforming growth factor beta binding site in betaglycan. Comparison with small extracellular matrix proteoglycans. J Biol Chem. 1993;268:22710–22715. [PubMed] [Google Scholar]
- Funderburgh JL, Hevelone ND, Roth MR, Funderburgh ML, Rodrigues MR, Nirankari VS, Conrad GW. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Visual Sci. 1998;39:1957–1964. [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Sinauer Associates Inc; Sunderland, MA: 2003. pp. 516–517. [Google Scholar]
- Glise B, Noselli S. Coupling of Jun amino-terminal kinase and decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997;11:1738–1747. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Eyelid development and fusion induced by cortisone treatment in mutant, lidgap-Miller, foetal mice. A scanning electron microscope study. J Embryol Exp Morphol. 1986;91:1–18. [PubMed] [Google Scholar]
- Hausser H, Groning A, Hasilik A, Schonherr E, Kresse H. Selective inactivity of TGF-beta/decorin complexes. FEBS Lett. 1994;353:243–245. doi: 10.1016/0014-5793(94)01044-7. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kao WWY, Kohno N, Nishihara-Hayashi M, Shiraishi A, Uno T, Yamaguchi M, Okamoto S, Maeda M, Ikeda T, Hamada H, Kondo K, Ohashi Y. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Visual Sci. 2004;45:2212–2217. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RL, Hall LS. Early development and embryology of the platypus. Philos Trans R Soc Lond, B Biol Sci. 1998;353:1101–1114. doi: 10.1098/rstb.1998.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JP, Weth K, De Souza SA, Junghans U, Muller HW, Hasenohrl RU. Facilitation of learning and long-term ventral pallidal-cortical cholinergic activation by proteoglycan biglycan and chondroitin sulfate C [in process citation] Neuroscience. 2000;100:355–361. doi: 10.1016/s0306-4522(00)00270-0. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Jester JV, Nicolaides N, Smith RE. Meibomian gland dysfunction: I. Keratin protein expression in normal human and rabbit meibomian glands. Invest Ophthalmol Visual Sci. 1989;30:927–935. [PubMed] [Google Scholar]
- Junghans U, Koops A, Westmeyer A, Kappler J, Meyer HE, Muller HW. Purification of a meningeal cell-derived chondroitin sulphate proteoglycan with neurotrophic activity for brain neurons and its identification as biglycan. Eur J Neurosci. 1995;7:2341–2350. doi: 10.1111/j.1460-9568.1995.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Mah DG, Benson A. The lidgap-Gates (lgGa) mutation for open eyelids at birth maps to mouse chromosome 13. Mamm Genome. 1996;7:403–407. doi: 10.1007/s003359900121. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Banks KG, Mah DG. Gaping lids, gp, a mutation on centromeric chromosome 11 that causes defective eyelid development in mice. Mamm Genome. 2000;11:440–447. doi: 10.1007/s003350010084. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconjugate J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY. The use of transgenic and knock-out mice in the investigation of ocular surface cell biology. Ocular Surf. 2003;1:5–19. doi: 10.1016/s1542-0124(12)70003-4. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY, Converse RL, Shiraishi A, Kao CW, Ishizaki M, Doetschman T, Duffy J. Keratin 12-deficient mice have fragile corneal epithelia. Invest Ophthalmol Visual Sci. 1996;37:2572–2584. [PubMed] [Google Scholar]
- Kelton DE, Rauch H. Linkage of open eyelids with linkage group VII of the mouse. J Hered. 1968;59:27–28. doi: 10.1093/oxfordjournals.jhered.a107633. [DOI] [PubMed] [Google Scholar]
- Koops A, Kappler J, Junghans U, Kuhn G, Kresse H, Muller HW. Cultured astrocytes express biglycan, a chondroitin/dermatan sulfate proteoglycan supporting the survival of neocortical neurons. Brain Res Mol Brain Res. 1996;41:65–73. doi: 10.1016/0169-328x(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Kresse H, Seidler DG, Muller M, Breuer E, Hausser H, Roughley PJ, Schonherr E. Different usage of the glycosaminoglycan attachment sites of biglycan. J Biol Chem. 2001;276:13411–13416. doi: 10.1074/jbc.M009321200. [DOI] [PubMed] [Google Scholar]
- Kurita R, Tabata Y, Sagara H, Arai K, Watanabe S. A novel smoothelin-like, actin-binding protein required for choroidal fissure closure in zebrafish. Biochem Biophys Res Commun. 2004;313:1092–1100. doi: 10.1016/j.bbrc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Li G, Gustafson-Brown C, Hanks SK, Nason K, Arbeit JM, Pogliano K, Wisdom RM, Johnson RS. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4:865–877. doi: 10.1016/s1534-5807(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Liang Y, Haring M, Roughley PJ, Margolis RK, Margolis RU. Glypican and biglycan in the nuclei of neurons and glioma cells: presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J Cell Biol. 1997;139:851–864. doi: 10.1083/jcb.139.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zhu G, Converse RL, Kao CWC, Nakamura H, Tseng SCG, Mui MM, Seyer J, Justice MJ, Stech ME, Hansen GM, Kao WWY. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–24636. [PubMed] [Google Scholar]
- Liu CY, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Corpuz LM, Conrad GW, Kao WW. The cloning of mouse keratocan cDNA and genomic DNA and the characterization of its expression during eye development. J Biol Chem. 1998;273:22584–22588. doi: 10.1074/jbc.273.35.22584. [DOI] [PubMed] [Google Scholar]
- Liu C, Arar H, Kao C, Kao WW. Identification of a 3.2 kb 5′-flanking region of the murine keratocan gene that directs beta-galactosidase expression in the adult corneal stroma of transgenic mice. Gene. 2000;250:85–96. doi: 10.1016/s0378-1119(00)00165-7. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Wilson SW. Pax proteins and eye development. Curr Opin Neurobiol. 1996;6:49–56. doi: 10.1016/s0959-4388(96)80008-0. [DOI] [PubMed] [Google Scholar]
- Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. Biglycan, a vascular proteoglycan, binds differently to HDL(2) and HDL(3): role of ApoE. Arterioscler, Thromb, Vasc Biol. 2001;21:129–135. doi: 10.1161/01.atv.21.1.129. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconjugate J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Reneker LW, Silversides DW, Patel K, Overbeek PA. TGF alpha can act as a chemoattractant to perioptic mesenchymal cells in developing mouse eyes. Development. 1995;121:1669–1680. doi: 10.1242/dev.121.6.1669. [DOI] [PubMed] [Google Scholar]
- Saika S, Shiraishi A, Saika S, Liu CY, Funderburgh JL, Kao CWC, Converse RL, Kao WWY. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Sartipy P, Bondjers G, Hurt-Camejo E. Phospholipase A2 type II binds to extracellular matrix biglycan: modulation of its activity on LDL by colocalization in glycosaminoglycan matrixes. Arterioscler, Thromb, Vasc Biol. 1998;18:1934–1941. doi: 10.1161/01.atv.18.12.1934. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Robenek H, Harrach B, Glossl J, Nolte V, Hormann H, Richter H, Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987;104:1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Snow AD, Kinsella MG, Parks E, Sekiguchi RT, Miller JD, Kimata K, Wight TN. Differential binding of vascular cell-derived proteoglycans (perlecan, biglycan, decorin, and versican) to the beta-amyloid protein of Alzheimer’s disease. Arch Biochem Biophys. 1995;320:84–95. doi: 10.1006/abbi.1995.1345. [DOI] [PubMed] [Google Scholar]
- Stein KF, Norris BE, Mason J. Development of an open eyelid mutant in mus musculus. Dev Biol. 1967;16:315–330. doi: 10.1016/0012-1606(67)90045-0. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Fujii S, Yoshida A, Shirasu Y. Morphological and genetic characteristics of the open-eyelid mutant spontaneously occurring in NC-strain mice. Jikken Dobutsu. 1988;37:455–462. doi: 10.1538/expanim1978.37.4_455. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Whinna HC, Choi HU, Rosenberg LC, Church FC. Interaction of heparin cofactor II with biglycan and decorin. J Biol Chem. 1993;268:3920–3924. [PubMed] [Google Scholar]
- Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- Zenz R, Scheuch H, Martin P, Frank C, Eferl R, Kenner L, Sibilia M, Wagner EF. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang W, Hayashi Y, Jester JV, Birk DE, Gao M, Liu CY, Kao WW, Karin M, Xia Y. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. EMBO J. 2003;22:4443–4454. doi: 10.1093/emboj/cdg440. [DOI] [PMC free article] [PubMed] [Google Scholar]