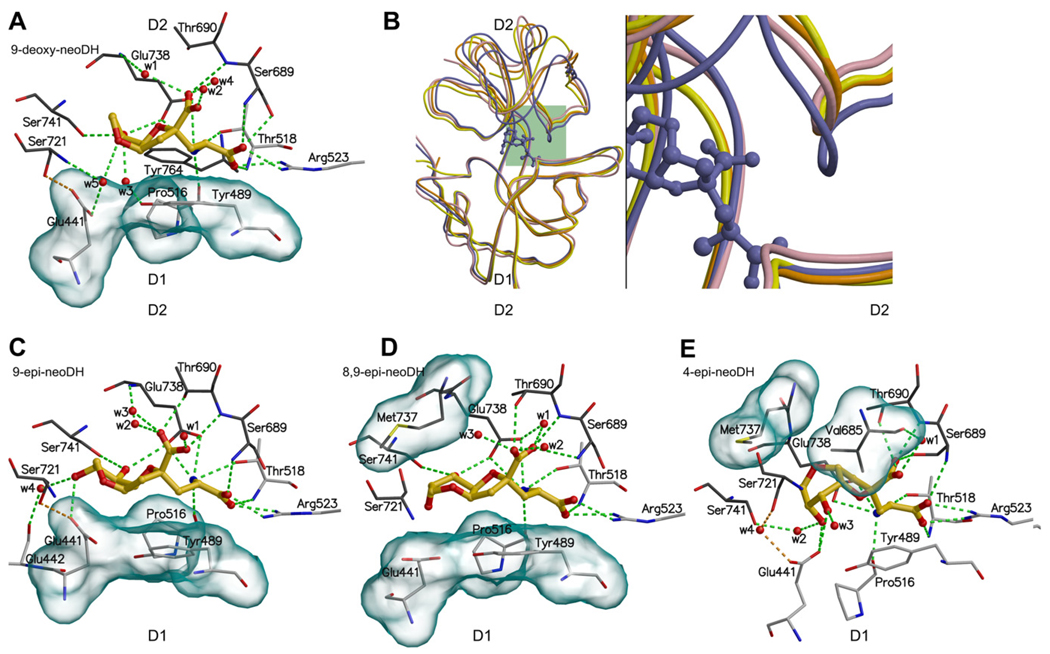
Fig. 6.
The binding modes of DH-based low affinity (or partial) agonist ligands for GluK1: (A) 9-deoxy-neoDH, (C) 9-epi-neoDH, (D) 8,9-epi-neoDH, and (E) 4-epi-neoDH. For coloring and interpretation (A, C–E) see Fig. 2B. In (B) is compared the crystal structures of two partial agonist–LBC complexes, GluA2–kainate (yellow) and GluK1–domoate (orange), agonist–LBC complex GluK1–DH (purple), and the MD simulated GluK1–9-deoxy-neoDH complex (pink). The structure comparison shows that 9-deoxy-neoDH is likely a partial agonist for GluK1, because its receptor-bound conformation resembles more the partial agonist structures than that of natural agonist DH (purple ball-and-stick).