Abstract
Objective
To identify characteristic anatomical features of the posterior compartment using MR cross-sectional anatomy and 3-D modeling.
Study Design
Supine, static proton-density MR images of 20 nulliparas were analyzed. MR images were used to create models in a selected exemplar.
Results
The compartment’s upper, mid, and lower segments are best seen in the axial plane. It is bounded inferiorly by the perineal body, ventrally by the posterior vaginal wall and dorsally by the levator ani muscles and coccyx. In the upper portion, the compartment is bordered laterally by the uterosacral ligaments while in the mid portion, there is more direct contact with the lateral levator ani muscles. In the lower portion, the contact becomes obliterated as the vagina and levator ani muscles become fused to one another and to the perineal body.
Conclusion
The posterior compartment has characteristic anatomic features in MR cross-sectional anatomy that can be further elucidated and integrated with 3-D anatomy.
Keywords: posterior compartment, cross-sectional anatomy, 3-D anatomy, levator ani, uterosacral ligaments
Introduction
Posterior vaginal wall prolapse, including the clinical problems of rectocele and enterocele, is a distressing and rarely discussed problem. Over 225,000 operations are performed annually for prolapse1 and 87% of those include surgery involving the posterior compartment2. Posterior compartment dysfunction is the least understood form of pelvic organ prolapse and many of the surgical treatments for it are empirically derived rather than based on objective, anatomic demonstration of structural abnormalities.
In 1999 we reported our early observations on posterior wall anatomy derived from the dissection of fresh and fixed cadavers, supplemented also by examination of histologic and macroscopic serial sections.3 Cadaveric dissections have demonstrated that the posterior compartment has well defined boundaries. These boundaries can be understood as a box with four sides where the top is open superiorly for the rectum and the recto-uterine pouch of Douglas (Figure 1). The cranial end of the vaginal wall is suspended by the uterosacral ligaments which extend below the peritoneum, and are seen in dissection and in cross sectional imaging.4 The distal end of the vagina fuses with the perineal body, while the lateral margins of the vagina form a visible line called the posterior arcus tendineus fascia pelvis.5 MR imaging and MRI based 3-D model reconstruction has allowed detailed anatomical study of living women that avoids many of the distortions that exist in cadaveric specimens. To date, however, MR has not been undertaken in studying the posterior vaginal wall.
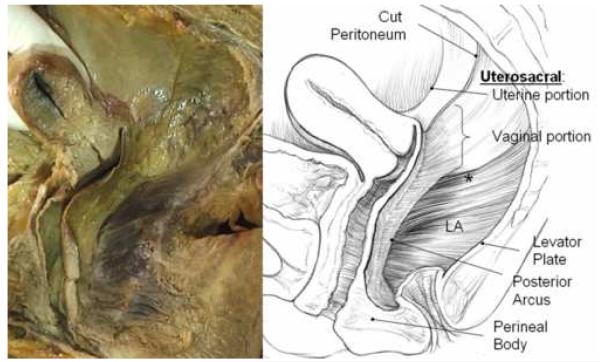
Figure 1.
Cadaver dissection (left) and illustration (right) of posterior compartment of a 56 year old multipararous female showing structural relationships after the rectum has been removed. Note the apical connections of the upper posterior vagina to the inside of the pelvic wall in a retroperitoneal position. These lie below the peritoneum and are dorsal and caudal to what is traditionally referred to as the uterosacral ligament. These structures are continuous with the posterior arcus tendineus fascia pelvis. At the distal end of the vagina the wall merges into the top of the perineal body. The lateral and dorsal margins of the compartment are formed by the levator ani muscles (LA) and the levator plate. The asterisk (*) denotes the region of the sacrospinous ligament overlain by the coccygeus muscle. © DeLancey 2007.
This purpose of this research is to evaluate posterior compartment anatomy in living, asymptomatic nulliparas using MR cross-sectional anatomy and 3-D model reconstruction. This type of information is needed so that MRI can be used as the basis for efforts to objectively identify specific structural defects in the posterior compartment in order to ultimately help guide research as well as clinical and surgical practices.
Methods
Twenty MR scans from the MR library at the University of Michigan were analyzed to identify and catalogue the key structures which clearly define the posterior compartment. These scans were those of asymptomatic nulliparas under the age of 50 who were recruited from the community by advertisements for study of pelvic floor anatomy and function not affected by vaginal birth. Inclusion criteria also included women older than age 18 years with no prior history of surgery for prolapse or incontinence. All subjects had uteri in situ. This sample size was chosen by the senior author based on prior experience with anatomic research as representing a sufficient number of scans to study features that are consistently present among normal women.
Static proton-density MR images were obtained in the supine position in axial, coronal and sagittal planes with a body coil. Multi-planar two-dimensional proton density pelvic MR images (TE 15 ms, TR 4000 ms) were obtained by use of a 1.5 T superconducting magnet (General Electric Signa Horizon LX, Milwaukee, WI). The field of view in axial and coronal images were both 16 × 16 cm and in the sagittal images 20 × 20 cm. All three acquisitions had a slice thickness of 4mm with a 1mm gap between slices.
The project was conducted in two phases. In phase one, detailed slice-by-slice examinations of axial, sagittal, and coronal images were conducted using the original source DICOM (Digital Imaging and Communications in Medicine) MR data in 5 subjects. Characteristic features of the posterior compartment components in each scan plane were catalogued. Next, in the additional 20 scans, the visibility of the characteristic features of the posterior compartment was tallied for all subjects and a plane specific percentage visibility was obtained.
For the second phase of the study, one exemplar MR dataset was selected. Using the 3-D slicer program (v 2.1b1, Brigham and Women’s Hospital, Boston, MA), tracings and 3-D models were made of the structures bounding the posterior compartment in the plane in which each structure was best visualized. Tracing of the structures and 3-D models were reviewed by the senior author. Each 3-D model was validated by overlaying the model with the original source image.
Results
All women were continent of stool and urine and had no symptoms of pelvic organ prolapse; the leading edge of pelvic organ descent was at least 1cm above the hymenal ring. The mean age of these subjects was 37.6 years (+/− 10.6 years) and eighteen of the 20 subjects (90%) were Caucasian. The mean BMI of subjects was 26.7 kg/m2 (± 5.4).
In phase I it was found that the posterior compartment could be divided into an upper, mid, and lower portion that mirrors the levels of support described for the vagina3. Each portion of the posterior compartment has characteristic MR features in the various scan planes in asymptomatic nulliparas (Table I).
Table I.
Posterior compartment characteristic features in each MRI scan plane
| Axial (Figure 2 panels) | Coronal (Figure 5 panels) | Sagittal (Figure 6 panels) | |
|---|---|---|---|
| Upper |
|
|
|
| Mid |
|
Lateral margins formed by pubovisceral and puborectalis muscles (−2.0 to −5.5) | |
| Lower |
|
Caudal margin formed by EAS (−5.5) and perineal body (−4.0) |
Perineal body well seen (L 0.5, 0, R 0.5, R1.0) |
Axial
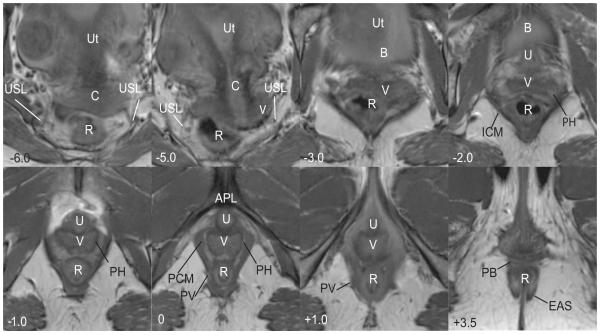
The axial scan plane shows the most MR details involving the posterior compartment (Figure 2). MR images are labeled in centimeters relative to the arcuate pubic ligament. Positive numbers are caudal to the ligament and negative numbers are cranial.
Figure 2.
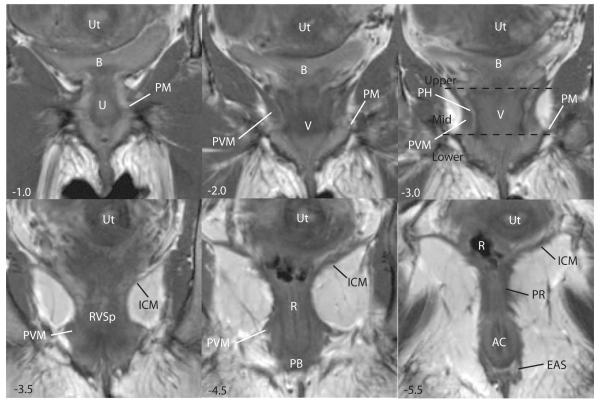
Axial scan of a nulliparous female showing various features of the posterior compartment. Images are presented cephalad (negative numbers) to caudad (positive numbers) in centimeters relative to the arcuate pubic ligament (APL). In the upper portion of the compartment (−6.0 to −2.0), there is loss of distinct separation of vagina (V) and surrounding structures; the bladder (B), iliococcygeus muscle (ICM) and uterus (Ut) are visible. At −6.0, the course of the uterosacral ligaments (USL) and the cervix (C) are visible. The course of the uterosacral ligaments (USL) is further delineated in −5.0. At −2.0 to 0.0 in the mid-portion of the compartment, the vagina has a “W” shape and is surrounded by a perivascular halo (PH); the pubococcygeus (PCM) and pubovaginalis (PV) are also visible. At +1.0, the pubovaginal division (PV) of the levator ani is still visible and the urethra (U) and arcuate pubic ligament (APL) become visible. In the lower portion of the compartment (+1.0) the vagina (V) takes on a “U” shape and fuses with the levator ani muscles. At +3.5 the perineal body (PB) is seen anterior to the rectum (R) and the external anal sphincter (EAS). © DeLancey 2007
The axial MR images can be divided into three portions: the upper, middle, and lower, each with distinct characteristics. The 3-D model (Figure 3) provides orientation of the relative locations of the upper, middle, and lower images which are shown in Figure 4. In the upper portion, the uterosacral ligaments form the lateral margins. The course of the uterosacral ligaments can be seen in 88% of the MR images analyzed and its visceral insertions on the cervix and vagina in 84%. The posterior compartment in the upper portion is bounded by the uterosacral ligaments on either side. The compartment is widest at this point and the contact between the genital tract and the pelvic walls is least direct in this region.
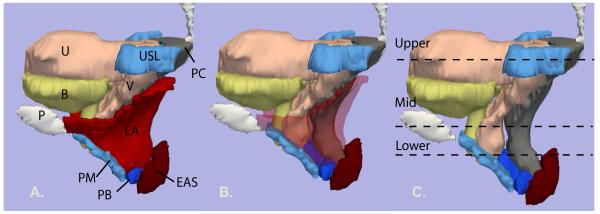
Figure 3.
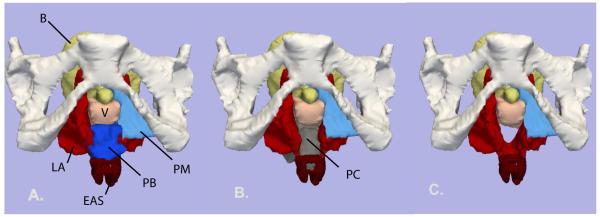
Left lateral view of the 3-D model. The outline of the mid-sagittal pubic bone (P) is shown. Bladder (B) – yellow; uterus (U) and vagina (V) – pink; uterosacral ligament (USL) and perineal membrane (PM) - turquoise blue; levator ani muscle (LA) – red; perineal body (PB) - royal blue; external anal sphincter (EAS) - dark red; posterior compartment (PC) - gray. Image A: all organs are shown. B: levator ani muscles have been faded to show underlying structures. C: levator ani muscles have been removed. The locations of the upper, mid and lower axial cross-sections in figure 2.2 are shown. © DeLancey 2007.
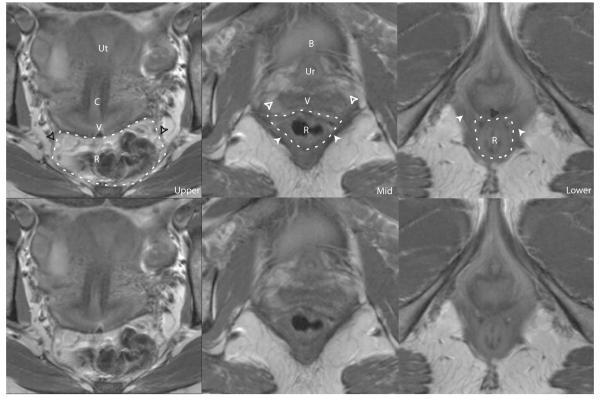
Figure 4.
Axial MR cross-sections of the levels of the posterior compartment. The compartment is outlined in a white dashed line. Upper: uterus (Ut), cervix (C), vagina (V), rectum (R), uterosacral ligaments (black arrowheads). Mid: urethra (Ur), perivascular halo (white unfilled arrows), pubococcygeus muscle (white filled arrows), bladder (B), urethra (Ur). Lower: pubovaginalis muscle (white filled arrows), rectovaginal space (grey unfilled arrow). The same images without labels are also shown. © DeLancey 2007.
In the mid portion, the shape of the posterior compartment becomes smaller with the posterior vaginal wall forming the ventral boundary, the pubococcygeus muscle forming the lateral margins and the sling from the puborectalis muscle forming the dorsal margin. Although the vagina in this portion is closer to the fibers of the levator ani muscles, a peri-vascular halo separates these structures.
In the lower portion of the posterior compartment, the peri-vascular halo is lost as the vagina becomes fused to the adjacent levator ani muscles, perineal body, and anus. The bottom of the compartment is marked where the perineal body and the subdivisions of the external anal sphincter can be seen. The perineal body was seen in 100% of the axial MR images analyzed.
Coronal
The transition from the upper, mid and lower segments of the posterior compartment can best be seen in the coronal scan plane (Figure 5, panel −3.0). In the upper portion, the uterosacral ligaments could only be seen in 40% of the coronal scans analyzed. Because of the wing like shape of the Iliococcygeus muscle and the posteriorly directed curve of the vagina, the posterior compartment appears to sit above the muscle in the coronal images. The lateral boundary of the posterior compartment in the mid portion is also best demonstrated in the coronal scan plane. In the mid portion, the fibers of the pubovisceral muscle, which includes the pubococcygeus, pubovaginalis and puborectalis muscles, are seen lateral to the vagina. Dorsal to the vagina, these fibers along with the fibers of the puborectalis muscle form the lateral margins of the posterior compartment. In the lower portion, the perineal body along with the external anal sphincter forms the caudal boundary of the posterior compartment; this could be seen in 92% of the scans analyzed (Figure 5, panel **−4.5). Portions of the perineal membrane, the connective tissue structure between the levator ani muscles and the vestibular bulbs, can also be seen as it forms lateral connections from the visceral organs and perineal body medially to the ischial pubic ramus laterally.
Figure 5.
Coronal scans of the same subject are depicted. Images are presented ventral (negative numbers) to dorsal (positive numbers) in centimeters relative to the APL . The bladder (B), uterus (Ut), urethra (Ur), vagina (V) are seen from −1.0 to −3.0. The fibers of the pubovisceral muscle (PVM) are seen in −2.0 to −4.5. At −1.0 to −3.0, the lateral portion of the perineal membrane (PM) is seen. At −3.0 the peri-vascular halo of the vagina is visible. At −3.5 the rectovaginal space (RVSp) and iliococcygeus muscle (ICM) are seen. The perineal body (PB) becomes visible at −4.5. At −5.5, the external anal sphincter (EAS) and puborectalis (PR) can also be seen as well as the anal canal (AC). The division of the upper, mid and lower portions of the posterior compartment are shown in panel −3.0. © DeLancey 2007.
Sagittal
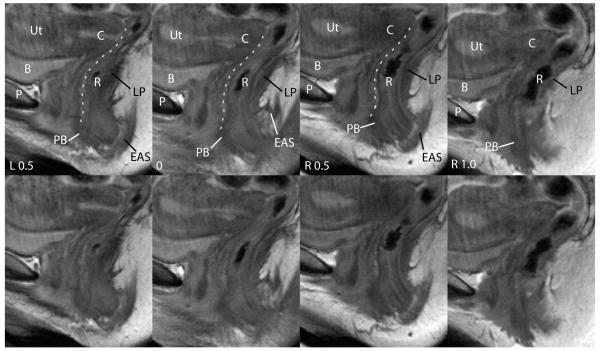
This scan plane best demonstrates the ventral and dorsal boundaries of the posterior compartment (Figure 6). The outline of the posterior vaginal wall which forms the ventral boundary is often well seen on multiple slices. The levator ani muscles from each side decussate to form the iliococcygeal raphé or levator plate in the midline. The levator plate forms the dorsal boundary of the posterior compartment and can be seen in all the sagittal images analyzed. The perineal body is also well seen in all the images as a dark, triangular structure between the posterior vaginal wall and the external anal sphincter.
Figure 6.
Sagittal scans of the same patient showing features of posterior compartment. The midline sagittal image is labeled 0 and other images left (L) or right (R) relative to it. The pubic symphysis (P), bladder (B), uterus (Ut), cervix (C) and rectum (R) are seen. The perineal body (PB) is visible anterior to the rectum and the external anal sphincter (EAS) as a low signal intensity triangular structure. The posterior wall of the vagina is outlined in white. The levator plate (LP) is also visible, forming the dorsal boundary of the posterior compartment. The same images without labels are also shown. © DeLancey 2007.
3-dimensional structural relationships
The posterior compartment has a complicated 3-dimensional shape where certain portions are parallel to one scan plane and oblique to others (Figure 3). The upper portion of the compartment has an oblique, dorsal-ventral orientation that follows the directionality of the iliococcygeal raphé. The mid portion of the posterior compartment is more aligned with the body axis as the sling of the levator ani compresses it against the pubic bone. 3-D modeling also demonstrates the relationship between the perineal body, perineal membrane, and the external anal sphincter complex in forming the caudal boundary of the posterior compartment (Figure 3 and 7). The perineal body, through fusion with the vagina ventrally, the perineal membrane laterally, and the external anal sphincter dorsally, forms a boundary that prevents forward and downward movement of posterior compartment (Figures 3 and 7).
Figure 7.
Inferior view of 3-D model with the pubic bones in place. A: Levator ani (LA) – red; Perineal membrane (PM) - turquoise blue; Perineal body (PB) – royal blue; External anal sphincter (EAS) – dark red; Bladder (B) – yellow; Vagina (V) – light pink. B: Bladder and perineal body have been removed to show the posterior compartment (PC) - grey. C: Posterior compartment model removed to show the space it occupies. © DeLancey 20
Comment
This project demonstrates that advanced MR imaging and 3-D modeling can provide a detailed anatomic depiction of the normal posterior compartment structures in asymptomatic, nulliparous, living women. It also illustrates clear posterior compartment boundaries provided by muscular and connective tissue structural components that can be reliably visualized in nulliparas. Our approach of using characteristic features in the various scan planes provides an inventory of anatomic findings and the frequency with which these features can be seen. It extends previous cadaver-based anatomical work by demonstrating a technique that can be used in living women.
There are several clinical implications of being able to directly image elements of the posterior compartment. Improved surgical outcomes have been achieved in other surgical disciplines through accurate anatomical identification of structural abnormalities as has been possible with lumbar disk disease or complex shoulder injury. Several steps are needed to accomplish this goal. First, it is necessary to identify the appearance of normal structures in imaging studies as we have done. Second, the appearance of different structural failures must be established in symptomatic women by comparing changes seen in their anatomy to normal. Third, the accuracy of the imaging modality to diagnose injuries is established through surgical exploration. Knowing normal MR cross-sectional and 3-D anatomy provides a guide to basic features of anatomy undisturbed by changes that occur during vaginal delivery; deviations and differences in women with defects of the posterior compartment can then be easily compared to normal anatomy. The list of normal features provided here can now be used to target areas for investigation that seeks to identify specific areas of structural failure.
The future application of these techniques to study symptomatic women with posterior wall failure can be expected to provide important information to guide surgical therapy and help understand operative failure. Directed repair of identified defects holds the theoretical promise of avoiding unnecessary surgical disruptions of normal areas and hopefully de novo complications such as the occurrence of postoperative dyspareunia6. MR studies also have the advantage of providing objective depictions of living women’s anatomy. Clinically, this may help in studying operative failure by providing a permanent record of anatomy that can be examined postoperatively and thus help identify preoperative anatomical factors that may be associated with operative failure. In addition, several different observers, blinded to subject complaint and clinical findings, can independently evaluate anatomy to test differing hypotheses using the same dataset. This may help improve on the poor inter-examiner and intra-examiner agreement that investigators have found with clinical exams for prolapse.7
Previous studies using surgical and cadaveric dissections have investigated the structures of the posterior compartment such as the rectovaginal septum,8 as well as its attachments to the pelvic sidewall5. Although our group has significant experience with cadaveric anatomical studies, we have found that MR analysis and 3-D models have added value; these analyses provide an inventory of normal characteristic cross-sectional and 3-D features in living women, in whom clinical symptoms and physical findings are known9. MR imaging has been used to study normal anatomy of the levator ani muscles and the anal sphincter10,11,12,13 but, not to our knowledge, the overall structural organization of the posterior compartment. While previous studies have used 3-D modeling techniques for studying the levator ani 14,15,16,17,18 and anal sphincter,19 this is also the first study, to our knowledge, to have built a model of the posterior compartment in its entirety. Our 3-D model helps to elucidate the complex interaction of the anatomy as a whole and thus, is valuable in the ultimate understanding of the posterior compartment structure and function. 3-D modeling demonstrated the boundaries of the posterior compartment as well as how specific MR cross-sectional anatomy related to each other in 3-D space.
In interpreting the results of our study, several limitations must be kept in mind. Our MR images are acquired with a 0.5 cm interval between images. This may account for why thin structures oblique to the scan plane, i.e. the uterosacral ligaments, were not seen in 100% of the images examined. While we do not think that this is a significant limitation for analysis of general aspects of normal anatomy, it may become more important in studying disruptions or defects in determining if a structure is actually missing or not imaged. The perineal body region, where localized defects in the fibromuscular layer of the posterior vagina occur as described by Richardson in 1995,20 and which resulted in changes in surgical treatment,21,22 are not well visualized at present. The newer, 3 Tesla magnets will allow good images with 0.2 cm slice intervals which should improve this situation in the near future. For our current projects, we are investigating the 3-T magnet as well as new protocols to improve our visualization of structures and overcome some current limits on visibility. In addition, at our current sample size we are not able to comment on racial differences that may exist in the posterior compartment anatomy due to the high proportion of Caucasians in our study sample.
While we are not currently advocating the routine use of MRI as a pre-operative imaging tool, based on our demonstrated ability to image normal features in this area, it seems likely in the near future that routine imaging may be warranted. This imaging may serve to objectively identify specific abnormalities in each of the regions of posterior vaginal wall support prior to surgical intervention. At present, defect identification depends on surgical dissection which does not allow for tailored treatment or surgical plan pre-operatively and requires dissection of areas that may not need repair. Pre-operative imaging studies may benefit patients in the same way that detailed MR imaging currently help orthopedic surgeons manage a complex knee injury23.
Condensation.
The normal posterior compartment has characteristic anatomic features that can be elucidated using MR imaging and 3-D modeling.
Acknowledgments
We gratefully acknowledge the support of the NIH through grant NIDDK R01 DK051405 and the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health and the National Institute of Child Health and Human Development 1 P50 HD044406.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Society of Gynecologic Surgeons 33rd Scientific Meeting, Orlando, Florida, April 2007.
Reprints will not be available.
References
- 1.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the united states, 1979-1997. Am J Obstet Gynecol. 2003;188(1):108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 2.Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: Five-year outcomes. Obstet Gynecol. 2006;108(2):255–63. doi: 10.1097/01.AOG.0000224610.83158.23. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–1728. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 4.Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JO. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;103(3):447–51. doi: 10.1097/01.AOG.0000113104.22887.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leffler KS, Thompson JR, Cundiff GW, Buller JL, Burrows LJ, Ybarra MA Schon. Attachment of the rectovaginal septum to the pelvic sidewall. Am J Obstet Gynecol. 2001;185(1):41–3. doi: 10.1067/mob.2001.116366. [DOI] [PubMed] [Google Scholar]
- 6.Cundiff GW, Fenner D. Evaluation and treatment of women with rectocele: focus on associated defecatory and sexual dysfunction. Obstet and Gynecol. 2004;104:1403–1421. doi: 10.1097/01.AOG.0000147598.50638.15. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside JL, Barber MD, Paraiso MF, et al. Clinical evaluation of anterior vaginal wall support defects: interexaminer and intraexaminer reliability. Am J Obstet Gynecol. 2004;191(1):100–4. doi: 10.1016/j.ajog.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Milley PS, Nichols DH. A correlative investigation of the human rectovaginal septum. Anat. Rec. 1968;163:443–452. doi: 10.1002/ar.1091630307. [DOI] [PubMed] [Google Scholar]
- 9.Hsu Y, Huebner M, Chen L, et al. Comparison of the main body of the external anal sphincter muscle cross-sectional area between women with and without prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007 doi: 10.1007/s00192-007-0340-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson MP, Lee RA, Berquist TH. Anatomy of anal sphincters and related structures in continent women studied with magnetic resonance imaging. Obstet Gynecol. 1990;7:846–51. doi: 10.1097/00006250-199011000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Hussain SM, Stoker J, Lameris JS. Anal sphincter complex: Endoanal MR imaging of normal anatomy. Radiology. 1995;197(3):671–7. doi: 10.1148/radiology.197.3.7480737. [DOI] [PubMed] [Google Scholar]
- 12.deSouza NM, Puni R, Kmiot WA, Bartram CI, Hall AS, Bydder GM. MRI of the anal sphincter. J Comput Assist Tomogr. 1995;19(5):745–51. doi: 10.1097/00004728-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Strohbehn K, Ellis JH, Strohbehn JA, DeLancey JO. Magnetic resonance imaging of the levator ani with anatomic correlation. Obstet Gynecol. 1996;87(2):277–85. doi: 10.1016/0029-7844(95)00410-6. [DOI] [PubMed] [Google Scholar]
- 14.Fielding JR, Dumanli H, Schreyer AG, Okuda S, Gering DT, Zou KH, et al. MR-based three-dimensional modeling of the normal pelvic floor in women: Quantification of muscle mass. AJR Am J Roentgenol. 2000;174(3):657–60. doi: 10.2214/ajr.174.3.1740657. [DOI] [PubMed] [Google Scholar]
- 15.Singh K, Jakab M, Reid WM, Berger LA, Hoyte L. Three-dimensional magnetic resonance imaging assessment of levator ani morphologic features in different grades of prolapse. Am J Obstet Gynecol. 2003;188(4):910–5. doi: 10.1067/mob.2003.254. [DOI] [PubMed] [Google Scholar]
- 16.Hoyte J, Jakab M, Warfield SK, et al. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gynecol. 2004;191(3):856–61. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 17.Margulies RU, Hsu Y, Kearney R, Stein T, Umek WH, DeLancey JO. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107(5):1064–9. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Y, Summers A, Hussain HK, Guire KE, Delancey JO. Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am J Obstet Gynecol. 2006;194(5):1427–33. doi: 10.1016/j.ajog.2006.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu Y, Fenner DE, Weadock WJ, DeLancey JO. Magnetic resonance imaging and 3-dimensional analysis of external anal sphincter anatomy. Obstet Gynecol. 2005;106(6):1259–65. doi: 10.1097/01.AOG.0000189084.82449.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson C. The anatomic defects in rectocele and enterocele. J Pelvic S. 1995;1(4):214–21. [Google Scholar]
- 21.Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: An analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171(6):1429–39. doi: 10.1016/0002-9378(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 22.Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179(6 Pt 1):1451–7. doi: 10.1016/s0002-9378(98)70009-2. [DOI] [PubMed] [Google Scholar]
- 23.Stoller D. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine. edn 2 Lippincott Williams & Wilkins; Philadelphia: 1997. [Google Scholar]