Abstract
Eumycetoma caused by Madurella mycetomatis is treated surgically and with high doses of ketoconazole. Therapeutic responses are poor, and recurrent infections are common. In search of therapeutic alternatives in the treatment of mycetoma, we determined the in vitro susceptibilities of M. mycetomatis isolates against caspofungin, anidulafungin, and micafungin. As a comparator fungus, Aspergillus fumigatus was used. Minimal effective concentrations (MECs) and MICs were assessed and compared to those of ketoconazole. M. mycetomatis isolates were not susceptible to the echinocandins.
Eumycetoma is a subcutaneous disease caused by a variety of microorganisms, both bacteria and fungi. The most common causative fungus is Madurella mycetomatis. After surgical debridement, eumycetoma is usually treated for extended periods of time with high doses of either itraconazole (ITZ) or ketoconazole (KTZ), which can result in hepatoxicity. In order to identify alternative antifungal therapies, the susceptibilities of M. mycetomatis to other antifungal agents (amphotericin B, 5-flucytosine, fluconazole, and voriconazole) have been determined before and compared to the obtained susceptibilities to ITZ and KTZ. M. mycetomatis remains most susceptible toward the azoles and amphotericin B; no activity was seen with 5-flucytosine (10).
The echinocandins are a relatively new class of antifungal agents, with caspofungin (CAS), anidulafungin (ANI), and micafungin (MICA) as its licensed representatives. Echinocandins inhibit the synthesis of 1,3-β-glucan, the main component of the fungal cell wall. In Candida spp., the echinocandins are fungicidal, but in molds such as Aspergillus species, the echinocandins show fungistatic activity. Limited activity has been noted against zygomycetes, basidiomycetes, and some Scedosporium species (12). Only one study addressed the susceptibility of M. mycetomatis to the echinocandins. In that study, the susceptibilities of only 3 isolates of M. mycetomatis against ANI were determined (6). No data are available for the other echinocandins.
We determined the in vitro susceptibilities of 17 clinical M. mycetomatis isolates to CAS, ANI, and MICA in comparison to the in vitro susceptibility of A. fumigatus ATCC 204305. All M. mycetomatis isolates were identified by internal transcribed spacer (ITS) sequencing. For M. mycetomatis, as a comparator, MICs were also determined for KTZ (Janssen Pharmaceuticals, Beerse, Belgium).
MICs were determined independently in triplicate in RPMI medium by using the previously reported 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assays for M. mycetomatis and A. fumigatus as described elsewhere (1, 10, 11). For A. fumigatus, conidia were exposed to the antifungal agents, while for M. mycetomatis, a hyphal inoculum was used, since this fungus does not usually sporulate on agar plates. In the past, hyphal inocula were also prepared for A. fumigatus, and it appeared that hyphal fragments show antifungal agent susceptibilities similar to those of conidia (11). The MIC endpoints for each antifungal agent were defined as the first concentrations resulting in a spectrophotometric reduction of more than 80%. The minimal effective concentration (MEC) endpoint was determined as the first concentration in which altered growth was noticed. Twofold-increasing drug concentrations were used, and they ranged from 0.016 mg/liter to 16 mg/liter for KTZ and 0.007 mg/liter to 128 mg/liter for CAS (Merck and Company, Rahway, NJ), ANI (Pfizer BV, Capelle aan de Ijsel, Netherlands), and MICA (Astellas Pharma, Leiderdorp, Netherlands). KTZ, CAS, and ANI were diluted in dimethyl sulfoxide (DMSO), and MICA was diluted in normal saline. The final concentration of DMSO per inoculum was as stated by the CLSI (2).
To determine the β-1,3-glucan concentration, microcentrifuge tubes were inoculated with 100 μl of an M. mycetomatis hyphal suspension in RPMI or an A. fumigatus conidial suspension in RPMI as described above. After incubation with the antifungal agents (7 days at 37°C for M. mycetomatis or 48 h for A. fumigatus), the mycelium was freeze-dried, and 250 μl of 1 M NaOH was added. This mixture was sonicated with a microprobe for 15 s at 26 μm and incubated at 52°C for 30 min. Glucan levels were determined by aniline blue fluorescence as described elsewhere, by using curdlan (Sigma) as a positive control (3, 8).
In accordance with previously published MICs for M. mycetomatis, all strains were strongly inhibited by KTZ, the drug of choice to treat eumycetoma in Sudan (Table 1). MICs for KTZ ranged from <0.016 mg/liter to 1 mg/liter. A concentration of 0.25 mg/liter was needed to inhibit the growth of 90% of the isolates (Table 1). Most of the M. mycetomatis strains were not inhibited in growth by the echinocandins (Table 1). Most MICs for CAS were 128 mg/liter, while the MICs of ANI and MICA were above 128 mg/liter (Table 1). As is seen in Table 1, only for isolate Mm41 were lower MICs obtained, and these were 16 mg/liter for CAS, 0.5 mg/liter for ANI, and 8 mg/liter for MICA. The results shown here are different from previously published susceptibility data for M. mycetomatis. In that study, the spores of three sporulating strains of M. mycetomatis were used. Conidia were harvested and exposed to ANI, and MICs of 1 mg/liter were obtained (6). The species M. mycetomatis is not well characterized, and in the past, misidentifications have occurred. One of the key features of this species is its lack of sporulation on agar plates. To ascertain that only M. mycetomatis isolates were used in the present study, all isolates were identified by ITS sequencing. None of our isolates did sporulate, and we therefore used hyphal fragments to determine the in vitro susceptibilities against the echinocandins. Our inoculation procedure, therefore, differs from that of Odabasi et al. (6), which could explain the discrepancy in results. Another explanation could be that the three isolates of Odabasi et al. resembled isolate Mm41, which in our study also appeared to be susceptible to anidulafungin. Since the isolates of Odabasi et al. were not used in our study, we cannot exclude this possibility.
TABLE 1.
Susceptibilities of M. mycetomatis and A. fumigatus to ketoconazole, caspofungin, anidulafungin, and micafungin
| Species | Strain | MIC (MEC) (mg/liter)a |
|||
|---|---|---|---|---|---|
| KTZ | CAS | ANI | MICA | ||
| M. | Mm31 | 0.063 | 64 | >128 | >128 |
| mycetomatis | Mm35 | 1 | 128 | >128 | >128 |
| Mm36 | 0.063 | 128 | >128 | >128 | |
| Mm39 | 0.031 | 64 | >128 | >128 | |
| Mm41 | 0.125 | 16 | 0.5 | 8 | |
| Mm43 | 0.063 | 128 | >128 | 128 | |
| Mm45 | 0.25 | 64 | >128 | >128 | |
| Mm46 | <0.016 | 64 | >128 | >128 | |
| Mm49 | 0.031 | 32 | >128 | >128 | |
| Mm50 | 0.063 | 128 | >128 | >128 | |
| Mm52 | 0.25 | >128 | >128 | >128 | |
| Mm54 | 0.031 | 64 | >128 | >128 | |
| Mm55 | 0.25 | 128 | >128 | >128 | |
| Mm64 | 0.063 | 64 | >128 | >128 | |
| Mm68 | 0.125 | 64 | >128 | >128 | |
| Mm73 | 0.063 | 64 | >128 | >128 | |
| Mm83 | 0.125 | 128 | >128 | 128 | |
| A. fumigatus | ATCC 204305 | ND | 128 (0.125) | >128 (<0.007) | >128 (<0.007) |
The in vitro antifungal susceptibilities of M. mycetomatis and A. fumigatus to ketoconazole (KTZ), caspofungin (CAS), anidulafungin (ANI), and micafungin (MICA) are shown. For all 17 M. mycetomatis isolates, the MICs are given; for the quality control A. fumigatus ATCC 204305 strain, both the MIC and the MEC (in parentheses) are given. ND, not done.
In the present study, Mm41 behaved different from the other M. mycetomatis isolates with regard to echinocandin susceptibility; it is the only isolate which shows some susceptibility toward the echinocandins, especially against ANI. Mm41 is not morphologically different from the other M. mycetomatis isolates and has the same cellular beta-glucan quantity as the other isolates. Furthermore, when this isolate was typed by selective amplification of restriction fragments (amplified fragment length polymorphism [AFLP]), this isolate clustered together with other M. mycetomatis isolates isolated from Sudan and used in this study (9).
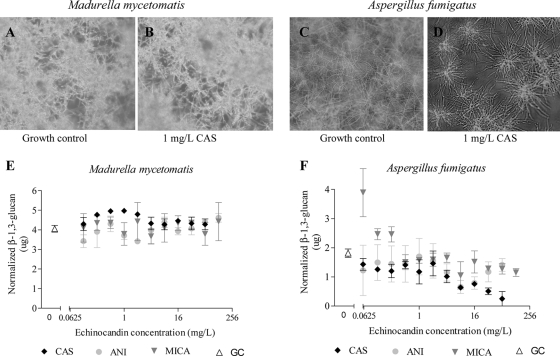
For A. fumigatus, growth was not completely inhibited by high concentrations of the echinocandins. Only at very high concentrations was lack of growth noticed (CAS [MIC of 128 mg/liter], ANI [MIC of 128 mg/liter], and MICA [MIC of >128 mg/liter]). At much lower concentrations, growth alteration was noted (MEC of 0.125 mg/liter for CAS and MECs of <0.03 mg/liter for ANI and MICA) (Fig. 1C and D). Therefore, it was investigated if alteration of growth also occurred in M. mycetomatis after exposure to the echinocandins. As shown in Fig. 1A and B, no growth alteration was observed under the tested conditions when M. mycetomatis was exposed to CAS, ANI, or MICA (the last two are not shown).
FIG. 1.
Effect of echinocandins on M. mycetomatis and A. fumigatus. (A) M. mycetomatis growth control. (B) M. mycetomatis exposed to 1 mg/liter CAS. (C) A. fumigatus growth control. (D) A. fumigatus exposed to 1 mg/liter CAS. (E) M. mycetomatis β-1,3-d-glucan concentration of strain Mm55 as determined by the aniline blue assay. β-1,3-d-Glucan concentrations were corrected to the number of viable cells with the XTT assay by the following formula: (amount of beta-glucan measured) × (number of viable cells in tested well/number of viable cells in growth control). Each point represents the mean β-1,3-d-glucan concentration with the standard deviation. (F) A. fumigatus β-1,3-d-glucan concentration as determined by the aniline blue assay. β-1,3-d-Glucan concentrations were corrected to the number of viable cells with the XTT assay. Each point represents the mean β-1,3-d-glucan concentration with the standard deviation. CAS, caspofungin; MICA, micafungin; ANI, anidulafungin; GC, growth control.
To confirm the lack of echinocandin activity against M. mycetomatis, β-1,3-glucan production was determined in M. mycetomatis and A. fumigatus. As shown in Fig. 1, under the experimental conditions, all three echinocandins were unable to inhibit β-1,3-d-glucan synthesis in M. mycetomatis. β-1,3-d-Glucan concentrations were documented for M. mycetomatis isolates not exposed to an echinocandin that were similar to those for M. mycetomatis isolates exposed to various echinocandin concentrations, even in ANI-inhibited isolate Mm41. In contrast, in A. fumigatus, the echinocandins did inhibit β-1,3-d-glucan synthesis as seen by the lowering β-1,3-d-glucan concentrations represented in Fig. 1F and reported by Kahn et al. (3).
From our results, it appears that M. mycetomatis is not susceptible to the echinocandin class of antifungal agents. The reason behind this intrinsic resistance was not explored in the present study, but some clues might be obtained from other fungi. Echinocandin agents are also ineffective against Fusarium species, Cryptococcus neoformans, and agents of zygomycosis. Resistance in Fusarium solani is shown to be partly caused by certain amino acid substitutions in the protein encoded by the target gene fks1 (4). Differences in the fks1 gene are not the only mechanism underlying echinocandin resistance. In the caspofungin-resistant fungus C. neoformans, the FKS enzyme itself was fully inhibited by low concentrations of CAS (5). Since the echinocandins require transport into the cell to their site of action, the surface properties of fungi might contribute to resistance. Since C. neoformans is highly melanized, it was hypothesized that this melanization could affect echinocandin susceptibility (7). For M. mycetomatis, the fks1 sequence is not known, but it has been demonstrated that the fungus can produce melanin both in vitro and in vivo. Further study is needed to determine the mechanism of this resistance.
In conclusion, in our assay, the echinocandins CAS, ANI, and MICA are not active against M. mycetomatis. There was no inhibition in growth, growth alteration, or reduction in β-1,3-glucan biosynthesis noted for M. mycetomatis isolates after exposure to these antifungal agents in the assays used. Therefore, the therapeutic potential of the echinocandins in the treatment of mycetoma infections caused by M. mycetomatis remains doubtful.
Acknowledgments
We have no transparency declarations to declare.
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Ahmed, A. O., W. W. van de Sande, W. van Vianen, A. van Belkum, A. H. Fahal, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2004. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob. Agents Chemother. 48:2742-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CLSI/NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, vol. 22. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 3.Kahn, J. N., M. J. Hsu, F. Racine, R. Giacobbe, and M. Motyl. 2006. Caspofungin susceptibility in Aspergillus and non-Aspergillus molds: inhibition of glucan synthase and reduction of beta-D-1,3 glucan levels in culture. Antimicrob. Agents Chemother. 50:2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katiyar, S. K., and T. D. Edlind. 2009. Role for Fks1 in the intrinsic echinocandin resistance of Fusarium solani as evidenced by hybrid expression in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 53:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maligie, M. A., and C. P. Selitrennikoff. 2005. Cryptococcus neoformans resistance to echinocandins: (1,3)beta-glucan synthase activity is sensitive to echinocandins. Antimicrob. Agents Chemother. 49:2851-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odabasi, Z., V. L. Paetznick, J. R. Rodriguez, E. Chen, and L. Ostrosky-Zeichner. 2004. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob. Agents Chemother. 48:1912-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shedletzky, E., C. Unger, and D. P. Delmer. 1997. A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal. Biochem. 249:88-93. [DOI] [PubMed] [Google Scholar]
- 9.van de Sande, W. W., R. Gorkink, G. Simons, A. Ott, A. O. Ahmed, H. Verbrugh, and A. van Belkum. 2005. Genotyping of Madurella mycetomatis by selective amplification of restriction fragments (amplified fragment length polymorphism) and subtype correlation with geographical origin and lesion size. J. Clin. Microbiol. 43:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Sande, W. W., A. Luijendijk, A. O. Ahmed, I. A. Bakker-Woudenberg, and A. van Belkum. 2005. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob. Agents Chemother. 49:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Sande, W. W. J., M. Tavakol, W. van Vianen, and I. A. J. M. Bakker-Woudenberg. 2010. The effects of antifungal agents to conidial and hyphal forms of Aspergillus fumigatus. Med. Mycol. 48:48-55. [DOI] [PubMed] [Google Scholar]
- 12.Zaas, A. K. 2008. Echinocandins: a wealth of choice—how clinically different are they? Curr. Opin. Infect. Dis. 21:426-432. [DOI] [PubMed] [Google Scholar]