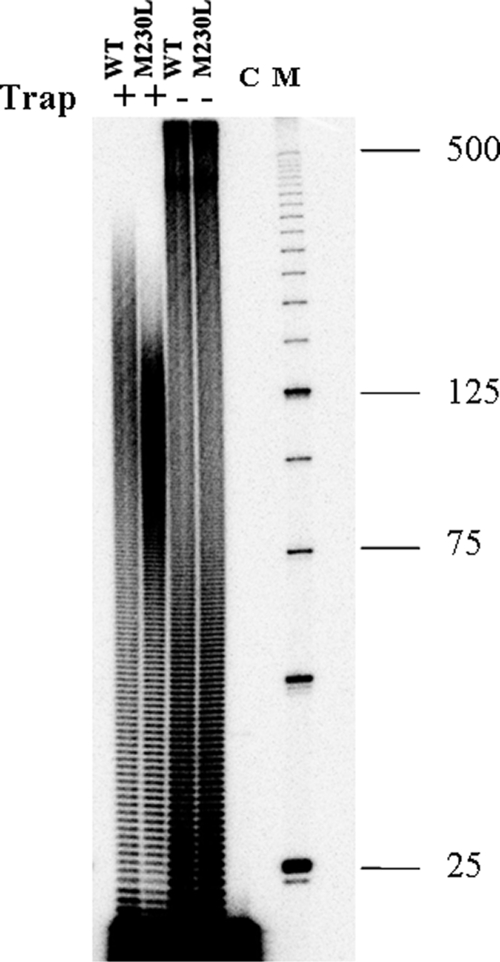
FIG. 5.
Reduced polymerase processivity of RT containing the M230L mutation. The processivities of the recombinant WT and M230L RT proteins were assessed with a homopolymeric RNA template poly(rA)-oligo (dT)12-18 DNA primer. The DNA primer was labeled with 32P at the 5′ terminus and was annealed to the poly(rA) RNA template at an equimolar ratio. Processivities were analyzed by monitoring the size distribution of the DNA products in fixed-time experiments in the presence of a heparin trap. Parallel reactions were run in the absence of the heparin trap to ensure that similar amounts of enzyme activities were present in the reactions. Lane C, control reaction to verify the efficiency of the heparin trap by preincubation with substrate prior to addition of RT; lane M, a 25-bp DNA ladder used as a size standard. The sizes (in kilobases) of some fragments of the standard are indicated on the right. All reaction products were resolved by denaturing 6% polyacrylamide gel electrophoresis and were visualized by phosphorimaging.