Abstract
The present study describes an accurate quantitative method for quantifying the adherence of conidia to the arthropod cuticle and the dynamics of conidial germination on the host. The method was developed using conidia of Metarhizium anisopliae var. anisopliae (Metschn.) Sorokin (Hypocreales: Clavicipitaceae) and engorged Rhipicephalus annulatus (Say) (Arachnida: Ixodidae) females and was also verified for M. anisopliae var. acridum Driver et Milner (Hypocreales: Clavicipitaceae) and Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) larvae. This novel method is based on using an organic solvent (dichloromethane [DCM]) to remove the adhered conidia from the tick cuticle, suspending the conidia in a detergent solution, and then counting them using a hemocytometer. To confirm the efficacy of the method, scanning electron microscopy (SEM) was used to observe the conidial adherence to and removal from the tick cuticle. As the concentration of conidia in the suspension increased, there were correlating increases in both the number of conidia adhering to engorged female R. annulatus and tick mortality. However, no correlation was observed between a tick's susceptibility to fungal infection and the amount of adhered conidia. These findings support the commonly accepted understanding of the nature of the adhesion process. The mechanism enabling the removal of the adhered conidia from the host cuticle is discussed.
The entomopathogenic fungus Metarhizium anisopliae var. anisopliae (Metschn.) Sorokîn (1883) infects a broad range of arthropod hosts and can be used as a biopesticide against different insect and tick species (8, 22, 35, 36). The adhesion of the conidia of entomopathogenic fungi to the host cuticle is the initial stage of the pathogenic process and includes both passive and active events (5, 10). The hydrophobic epicuticular lipid layer plays an important role during both the attachment process and the germination of the conidia on the surface of the host (15, 19). According to Boucias et al. (7), the attachment of conidia to the host cuticle is based on nonspecific hydrophobic and electrostatic forces. The conidia of most entomopathogenic fungi, including M. anisopliae, have an outer cell layer made up of rodlets (6). The hydrophobins, specific proteins present in the rodlet layer, mediate the passive adhesion of conidia to hydrophobic surfaces, such as the cuticles of arthropods (16, 45, 46). However, as germination commences, the hydrophobins are replaced by an adhesion-like protein, Mad1, which promotes tighter and more-specific adhesion between the conidia and the host (44). Many factors may affect the adhesion and persistence of conidia on the host cuticle (i.e., characteristics of the pathogen, including its virulence [2, 18, 48], conditions under which the pathogen is cultured [17], type of spores [7, 16], topographical and chemical properties of the host cuticle [9, 38, 42], host surface hydrophobicity [15, 23], host behavior [31, 33], and environmental conditions [33]). Conidia of M. anisopliae have shown an affinity to cuticular regions containing setae or spines (7, 38) and to highly hydrophobic cuticle regions, such as mosquitoes' siphon tubes (23) and intersegmental folds (43). Sites with higher numbers of adhered conidia varied among host species. However, in general, the membranous intersegmental regions were often particularly attractive sites for conidial attachment (26). Variation in the distribution of conidia across different anatomical regions has also been noted in studies of several tick species inoculated with entomopathogenic fungi (3, 21, 22). An evaluation of the attachment of Beauveria bassiana conidia to three tick species, Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis, demonstrated that the distribution patterns of the different conidia on the ticks' bodies were not uniform (22). The density of the conidia and their germination varied dramatically across different anatomical regions of Amblyomma maculatum and A. americanum that had been inoculated with B. bassiana (21). Arruda et al. (3) demonstrated that mass adhesion of M. anisopliae conidia to engorged Boophilus microplus females occurs predominantly on ticks' legs, suggesting its association with the presence of setae.
There are a few approaches for assessing the adhesion of conidia to the host cuticle that are based on direct observation of the conidia on the arthropod cuticle. They involve examining a few areas on the surface of an arthropod by means of scanning electron microscopy (SEM) (11, 15, 30), transmission electron microscopy (TEM) (4), or fluorescence microscopy following vital staining of the conidia (2, 28, 29, 37). These methods are expensive, time-consuming, and relatively inaccurate due to the uneven distribution of conidia on the host surface.
In this work, we describe a quantitative method for determining the total amount of conidia that have adhered to a whole host cuticle. This method is based on removing adhered conidia from the tick cuticle using an organic solvent, separating the conidia from the extract by centrifugation, resuspending the conidia in a detergent solution, and then counting the conidia in a hemocytometer. The efficacy of the method was evaluated by comparing the results of this procedure with those of a supplementary examination of conidial removal using SEM.
The term “adhered” is often used to define conidia in different states: washed or unwashed after inoculation, present on the host cuticle immediately after inoculation, or kept for several hours (1, 2, 38). In this paper, the term “adhered conidia” refers to conidia that remained on the cuticle after washing by vortexing the inoculated and dried host in an aqueous solution of Triton X-100 and rinsing of the material under tap water.
MATERIALS AND METHODS
Rearing the arthropods.
Rhipicephalus annulatus (Say) (Arachnida, Ixodidae) ticks were collected in 1984 from cattle in Israel and fed every 2 months on healthy 1- to 3-month-old Friesian calves. Eggs and unfed larvae were maintained at 28°C and 85% relative humidity (RH). Hyalomma excavatum (Koch) ticks were obtained from a colony that has been maintained in the laboratory since 1992. Their preimaginal stages were maintained on gerbils (Meriones tristrami), and their adult stages were maintained on rabbits. The nonfeeding stages were maintained at 28°C and 85% RH. After completion of the prefeeding period, the ticks were stored at 14°C until use.
Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) larvae of a laboratory colony were fed a diet comprised of starter ration (starter mash) (50%), ground wheat (25%), ground dog food pellets (Nutra Nugget; Meta, Missouri) (20%), and yeast (5%), all dry weight, to which 5% (wt/vol) of water was added. The colony was kept under a 28°C, 12-h light/12-h dark regime.
Preparation of fungi.
M. anisopliae var. anisopliae strain 7 was isolated in Israel in 1998 from an unidentified coleopteran. It was found to be very virulent to many species of tick instars (14). For this reason, this isolate was chosen for our comparison of the adhesion of conidia to the susceptible versus resistant hosts, R. annulatus and H. excavatum, respectively.
Metarhizium anisopliae var. acridum strain 5 (kindly provided by G. Zimmerman) was isolated in Madagaskar in 1992 from Locusta migratoria. In previous experiments, it was observed to be a low-virulence isolate for R. annulatus engorged females (13). A month before the beginning of the experiment, these isolates were reisolated from laboratory-infected engorged female R. annulatus on Sabouraud dextrose agar (SDA) (Difco, Becton Dickinson, Sparks, MD) supplemented with ampicillin (100 μg per ml; Sigma, Israel) and dodine pestanal (100 μg per ml; Sigma, Israel). To obtain the conidia, the fungus was grown on SDA for 2 weeks at 25°C. Conidia were harvested by scraping the fungal colony, suspending the collected material in 10 ml sterile distilled water containing 0.01% Triton X-100, and then vortexing and sonicating for 5 min (Ultrasonic Cleaner D80H; Chemist Co., Taipei Hsien, Taiwan) to break up the clumps of conidia. After sonication, the suspension was filtered through Miracloth (Calbiochem; La Jolla, CA), and conidial concentrations were determined using a hemocytometer. The suspension was adjusted to the required concentrations. The percentage of viable conidia had been previously determined on SDA, and conidia with germination rates of 95 to 100% were used.
Evaluation of conidial adhesion to the tick cuticle.
The method was developed using conidia of M. anisopliae var. anisopliae strain 7 and engorged R. annulatus females. M. anisopliae var. acridum strain 5 and engorged R. sanguineus females and A. diaperinus larvae were tested in the final stage of the study.
(i) Inoculation of arthropods and elimination of nonadhered conidia.
Engorged female ticks were washed with tap water, allowed to air dry, and then used for the adhesion experiments. We inoculated the ticks with M. anisopliae var. anisopliae strain 7 by dipping them individually in conidial suspensions (2 ml per tick) of various concentrations for 5 s. The inoculated ticks were air dried on mesh (mesh size, 2 mm) at room temperature until they were completely dry. The amount of time that conidia were exposed to the tick cuticle varied from 2 to 24 h. After this period, the inoculated ticks were washed in a vortex for 5 s with water containing 0.01% Triton X-100 and then rinsed with tap water. These actions eliminated any conidia that had not really adhered to the cuticle but were only resting on the cuticle surface. After rinsing, the ticks were left to dry on filter paper for 2 h. A. diaperinus larvae were inoculated with M. anisopliae var. anisopliae strain 7 by the same method.
(ii) Removing and counting adhered conidia.
We compared the use of two different solvents for removing adhered conidia from tick cuticle: (i) a nonpolar solvent with a polarity index of 0, n-pentane (99.9%; Uvasol, Merck, Germany), and (ii) a solvent with a polarity index of 3.1, dichloromethane (DCM) (99.9% GC grade; Bio-Lab Ltd., Israel). Each tick was placed in a 15-ml polypropylene centrifuge tube (1 tick per tube; Corning, Inc., Corning, NY) containing the solvent, and the tube containing the tick and the solvent was then shaken by hand for 5 min. The adhesion of the conidia to the tubes had been tested previously and found to be negligible. The average tick surface area was roughly calculated as if it were the same shape as a box: 2(ℓb + bh + hℓ), where ℓ is the height of the object and b and h are the lengths of its sides. Ten ticks of each species were used for surface area calculations. Because the average surface areas of the two tick species differ, the solvent volume per tick was adjusted accordingly: 1 ml of solvent for each R. annulatus tick and 2.3 ml for each H. excavatum tick. The surface area of eight A. diaperinus larvae was equal to that of one engorged R. annulatus female, and this proportion was used to determine the appropriate solvent volumes.
To evaluate the influence of the duration of solvent extraction on conidial removal, the procedure was applied for 5, 10, and 20 min.
Subsequently, the solvent extract containing the conidia was transferred to a new tube and mixed with ethyl alcohol (1:1) (99.8% analytical; Frutarom Ltd., Haifa, Israel) and centrifuged for 30 min at 6,000 × g (fixed-angle rotor model RS 300; centrifuge model NF800; Nuve, Ankara, Turkey) at a temperature of 25°C. The supernatants were examined under a light microscope, and the presence of conidia in these supernatants was found to be negligible. The conidium-free supernatants were discarded, and each of the conidium-containing sediments was mixed with 1 ml of sterile distilled water with 0.1% Triton X-100. The tubes were vigorously shaken, and the conidium titer was examined with a hemocytometer. Additional details concerning this method are presented in Results.
The germination rate of the conidia that had adhered and were then removed was evaluated by counting the germinated and nongerminated conidia directly in a hemocytometer.
(iii) Confirming removal of adhered conidia: SEM observation.
Engorged R. annulatus females and A. diaperinus larvae were inoculated with a suspension containing 1 × 108 conidia per ml as described above. They were fixed in a solution of glutaraldehyde (2.5%) in phosphate buffer (0.1 M, pH 7.2). After 2 h, the samples were rinsed repeatedly with phosphate buffer. The samples were rinsed every 10 min over the course of 1 h (five to six times). The samples were dehydrated in increasing concentrations (25%, 50%, 75%, 95%, and 100%) of ethanol and dried in a critical point dryer ((Bio-Rad CPD 750; Hemel, Hempstead, United Kingdom) and mounted on metal stubs. The samples were coated with gold and observed under a JEOL-JSM5410LV scanning electronic microscope (Tokyo, Japan) (20 kV).
Bioassay.
We inoculated female ticks by dipping them into a conidial suspension (1 × 107 conidia per ml) for 10 s. The inoculated ticks were then transferred to dishes lined with moist filter paper (5 females per dish) and incubated at 25°C and nearly 100% RH for 14 days. Daily tick mortality was recorded, and the dead ticks were removed from the dishes. Each test was repeated two to three times, with at least 25 females each time. Prior to each bioassay, conidial viability was determined by seeding SDA with conidia, incubating the culture at 25°C for 24 h, and then counting the percentage of germinating conidia. Only suspensions with germination rates of at least 95% were used.
Data analysis.
The nongerminated and germinated conidia 24 h postinoculation (p.i.) and the live and dead ticks from the bioassay experiments were counted, and percentages of germination and mortality were calculated. Results are presented as means plus standard deviations (SD). To evaluate the differences between the adhesion of conidia to the cuticles of resistant ticks compared to that for susceptible ticks, t tests were used.
RESULTS
Method development.
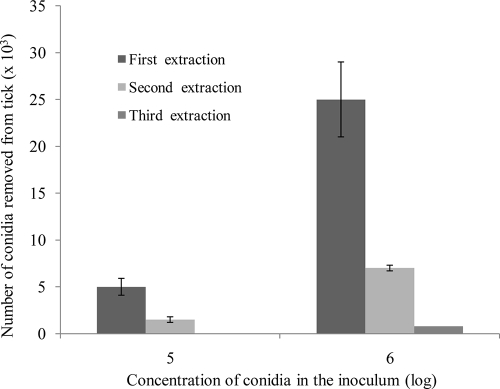
A preliminary comparison of the numbers of adhered conidia that were removed from tick cuticles by solvents showed that the DCM extract contained about 10 times more conidia than the pentane extract. Five-minute pentane extraction of ticks that had been inoculated with a 1 × 106-conidia-per-ml suspension removed (2.0 ± 1.2) × 103 conidia per tick on average, whereas 5-min DCM extractions yielded (2.23 ± 3.7) × 104 conidia per tick, on average. For both solvents, increasing the duration of the extraction (5, 10, or 20 min) did not increase the number of conidia removed; the average number of conidia in the pentane extract was (2.0 ± 1.2) × 103 for the 5-min extraction and (2.2 ± 0.8) × 103 for the 20-min extraction. In the DCM extract, the average numbers of removed conidia were (2.2 ± 0.4) × 104 and (2.7 ± 0.8) × 104, accordingly. Since a 10-min extraction was more practical than a 5-min extraction for simultaneous treatment of a large number of probes, it was used for all subsequent assays. To determine how thorough the removal of the conidia from the tick cuticle by DCM truly was, ticks were repeatedly immersed in fresh portions of DCM for 10 min at a time for second and third extractions. The data presented in Fig. 1 demonstrate that the first DCM extraction removed about 80% of the adhered conidia from the tick cuticle. After two successive extractions, 98 to 100% of the adhered conidia had been moved from the tick cuticle to the solvent. The success of removing the adhered conidia using this method was confirmed by SEM observations (described below).
FIG. 1.
Efficacy of successive DCM extractions for the removal of adhered conidia of M. anisopliae var. anisopliae strain 7 from tick cuticle. Engorged R. annulatus female ticks were inoculated with suspensions of conidia (105 or 106 conidia per ml). The adhered conidia were removed with 1 ml DCM; duration of solvent contact with tick was 10 min. Bar, means ± standard errors (SE).
Influence of exposure period on adhesion of conidia to the tick cuticle.
During the first hour following the ticks' inoculation with the conidial suspension, the ticks were still wet. Therefore, the influence of the duration of the ticks' exposure to the conidia on conidial adhesion was evaluated starting 2 h p.i., by which time the ticks were completely dry. The average number of adhered conidia was not significantly affected by the different exposure periods and varied from 2.0 × 104 to 3.1 × 104 conidia per tick (Table 1). However, following exposure periods longer than 2 h, the coefficient of variation of conidium removal increased 3 to 7.5 times. Also, beginning 6 h p.i., we observed some germinated conidia. The percentage of germinated conidia in the DCM fraction increased from 0.9% ± 0.5% at 6 h p.i. to 18.7% ± 4.4% at 24 h p.i.
TABLE 1.
Influence of duration of exposure of M. anisopliae var. anisopliae strain 7 conidia to surfaces of engorged R. annulatus females on numbers of conidia that adhered to tick cuticlesa
| Parameter | Value at time (h)b: |
|||||
|---|---|---|---|---|---|---|
| 2 | 6 | 8 | 12 | 18 | 24 | |
| Avg no. of conidia adhering to each tick, ×104 (SD) | 2.6 (0.2) | 3.1 (1.2) | 2.0 (0.6) | 2.2 (1.5) | 2.0 (0.7) | 2.5 (1.2) |
| Coefficient of variation | 9.16 | 38.02 | 32.20 | 69.33 | 35.15 | 46.85 |
Ticks were inoculated with a suspension with a concentration of 1 × 106 conidia per ml.
Hours of exposure of conidia to tick cuticle.
Efficacy of removal process examined by SEM.
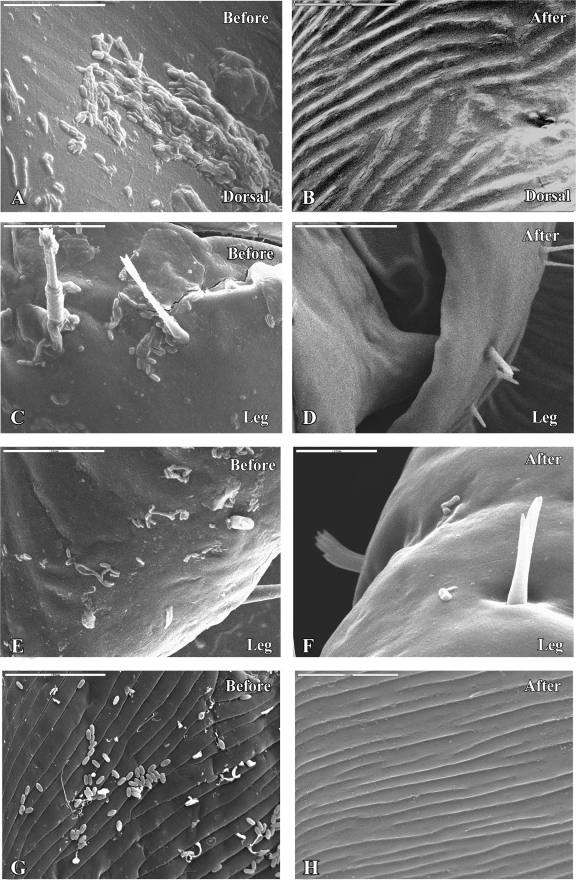
The successful removal of adhered conidia using DCM was confirmed by SEM observations. Figures 2 A and C show M. anisopliae var. anisopliae strain 7 conidia that adhered to the tick cuticle and remained on the cuticle surface after 2 h of incubation at 25°C and 100% RH. The surfaces of the ticks' bodies were covered with individual conidia, small groups of conidia, short chains, and large aggregates. The conidia were attached to the ticks' body surfaces, with no preference for specific sites such as seta-rich or grooved areas. The photograph in Fig. 2E depicts inoculated tick cuticle 24 h p.i., at which point about 30% of the adhered conidia had started to germinate. The germinated conidia produced both short and long germ tubes, and we did not observe any relationship between germ tube length and anatomical region.
FIG. 2.
(A to D) Electron micrographs of engorged R. annulatus female inoculated with M. anisopliae var. anisopliae strain 7 (1 × 108 conidia per ml) before (A, C) or after (B, D) treatment with DCM, 2 h p.i. Scale bars, 50 μm. (E and F) Engorged R. annulatus female inoculated with M. anisopliae var. anisopliae strain 7 (1 × 108 conidia per ml) before (E) or after (F) treatment with DCM, 24 h p.i. Scale bar: 50 μm (E) or 20 μm (F). (G and H) A. diaperinus larvae inoculated with M. anisopliae var. anisopliae 7 (1 × 108 conidia per ml) before (G) or after (H) treatment with DCM 2 h p.i. Scale bar, 50 μm.
Figures 2B, D, and F show the surfaces of ticks that were inoculated with a suspension of M. anisopliae var. anisopliae strain 7 conidia and incubated for 2 or 24 h under the conditions discussed previously but were washed with DCM). Washing the conidia with DCM 2 h p.i. removed all adhered conidia (Fig. 2B and D) from the tick cuticle. However, the same procedure used 24 h p.i. removed both germinated and nongerminated conidia but not those that had formed appressoria (Fig. 2F). The number of these remaining conidia was very low and thus can be disregarded in the evaluation of the success of the conidium extraction procedure.
This method for conidium removal was also examined on ticks inoculated with M. anisopliae var. acridum strain 5 (data not shown) and on A. diaperinus larvae inoculated with M. anisopliae var. anisopliae 7. SEM observations confirmed that the method is effective in these cases as well. Figure 2 shows M. anisopliae var. anisopliae strain 7 conidia attached to the cuticles of A. diaperinus larvae before (G) and after (H) extraction.
Effect of conidial concentration on conidial adhesion to the tick cuticle and tick mortality.
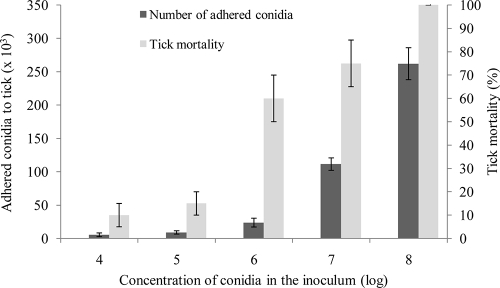
The adhesion of M. anisopliae var. anisopliae strain 7 conidia to the tick cuticle 2 h p.i. depended on the concentration of the conidial inoculum and was strongly correlated (correlation coefficient, 0.86) with tick mortality (Fig. 3). At low conidial concentrations (1 × 104 to 1 × 105 conidia per ml), the number of adhered conidia varied from 5.5 × 103 to 9.0 × 103 conidia per tick and tick mortality was 10 to 15% on day 12 p.i. A higher mortality rate (60 to 100%) was observed for ticks that were inoculated with suspensions of 1 × 106 to 1 × 108 conidia per ml. In these cases, the rate of conidial adhesion reached (25 to 260) × 103 conidia per tick.
FIG. 3.
Number of M. anisopliae var. anisopliae strain 7 conidia adhering to R. annulatus cuticle and tick mortality at inoculation in suspension of different conidial concentrations, 14 days p.i. Bars represent means ± standard errors.
Adhesion of M. anisopliae conidia to susceptible and resistant ticks.
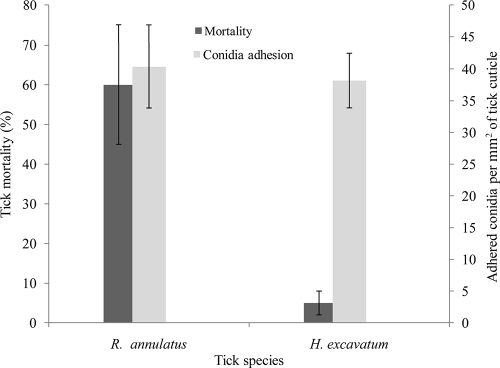
We compared the adhesion of conidia to susceptible (R. annulatus) and resistant (H. excavatum) engorged female ticks. As was shown above, the mortality of R. annulatus inoculated with M. anisopliae var. anisopliae strain 7 (1 × 106 to 108 conidia per ml) reached 60 to 100%, while H. excavatum females were very resistant to M. anisopliae (14). Only one or two H. excavatum females out of 25 inoculated ticks died during the 2-week bioassay period, regardless of the concentration of conidia in the inoculum (106 to 108 conidia per ml). The mortality rate in the noninfected control group was 0 to 5% for both tick species.
About three times more conidia were attached to the cuticles of the engorged H. excavatum female than to R. annulatus females (28,750 ± 7,077 and 11,300 ± 3,363, respectively). However, the cuticle surface of H. excavatum was about three times larger than that of R. annulatus (753 ± 85 and 280 ± 60 mm2, respectively). Thus, the number of adhered conidia per mm2 of tick cuticle did not differ significantly (df, 8; T, 1.84; P = 0.1) between the two ticks (Fig. 4). Moreover, neither the number of conidia per cm2 of tick cuticle nor the total number of conidia that adhered to each tick was related to the relative resistance of the ticks to M. anisopliae (Fig. 4).
FIG. 4.
Tick mortality and number of M. anisopliae var. anisopliae strain 7 conidia adhering to two tick species. Bars represent means ± standard deviations. Concentration of conidia in inoculum, 106 per ml; bioassay period, 12 days.
DISCUSSION
The aim of the current research was to investigate the process of adhesion of M. anisopliae conidia to the tick cuticle and to compare the numbers of conidia that adhered to susceptible and resistant ticks. Since the commonly used methods for evaluating adhesion, which are based on direct microscopic observation of conidia on different host sites, are inaccurate, we developed a quantitative assay based on removing all conidia that have adhered to the arthropod cuticle and counting them in a hemocytometer.
Earlier attempts to remove adhered conidia from cuticles of insect larva hosts by boiling the infected larvae in detergent or a mixture of chloroform and methanol or by soaking the material in 0.1 M NaOH, 0.1 M HCl, or cell wall-dissolving enzymes failed to remove significant numbers of attached conidia (7). However, M. anisopliae var. acridum conidia have been successfully removed from locust wings using a 2% solution of laminarinase obtained from Trichoderma spp. (20). In this study, the proposed method includes the following steps: (i) use of DCM to eliminate the ability of conidia to adhere to the tick cuticle; (ii) sedimentation of conidia by suspending them in the DCM extract through the addition of ethyl alcohol and following centrifugation; (iii) mixing of the conidial sediment with an aqueous detergent solution; and (iv) counting of conidia using a hemocytometer. This method was found to be simple, relatively quick, and easy to implement. Its efficiency was confirmed by SEM observations, and as was directly observed, DCM removed practically all adhered conidia.
The mechanism of how the DCM removes the adhered conidia is not clear. One of the two following theories may be correct. The characteristics of the solvents used to release the hydrophobic conidia from the host surface play an important role in the proposed method and may also shed some light on the interaction between the conidia and the host surface. Both epicuticular lipids and proteins (hydrophobins and adhesins) (44, 46) are expected to play roles in this interaction. The epicuticular components of R. annulatus are unknown, but according to Gilby (12), the epicuticular wax layer of R. microplus consists mainly of cholesterol derivatives, saturated acids, and alcohols with chains of approximately 30 carbons. The fact that adhered conidia were hardly removed with pentane but were efficiently extracted with dichloromethane (DCM), which is a more polar solvent, can be explained by the fact that DCM is more efficient at dissolving the epicuticular long-chain acids and alcohols than the nonpolar pentane (32). Thus, it is possible that in the applied method, the polar wax compounds involved in the adhesion of the conidia are removed from the epicuticle, thereby releasing the adhered conidia. Dichloromethane is also known as a protein-denaturing solvent (34). Since the adhesion of hydrophobic conidia to the hydrophobic host cuticle is mediated by proteins, DCM might release the adhered conidia by denaturing and/or inactivating these proteins.
Following the addition of ethanol and centrifugation, the conidia were concentrated in the sediment and the obtained supernatant was conidium free; the conidia did not adhere to the centrifuge tube and were successfully submersed in the detergent solution. There are two possible reasons for the inability to receive conidial sediment in the DCM alone. First, the conidia floated on the surface of the relatively high-density DCM (1.3266 g/cm3). Mixing DCM with ethyl alcohol (0.789 g/cm3) reduced the density of the solvent solution, bringing it closer to that of water and thereby facilitating the sedimentation of the conidia. The second possible reason involves the hydrophobic nature of the conidia, which prevented their distribution in DCM, which is a relatively polar solvent. Ethyl alcohol, which is miscible in DCM, served as a cosolvent and a surface active agent. When it was added to the extraction mixture, it may have enabled DCM to surround the hydrophobic conidia, suspending them in the solvent mixture.
In our studies of the effect of the time after inoculation on adhesion, the coefficient of variation was under 10% at 2 h p.i. but increased with time to be greater than 30% at 6 h p.i. This increase in variation can be explained by the mechanical loss of conidia due to the activity of the ticks, as has been demonstrated for some insects (2). This explanation is particularly plausible, since, as observed by SEM, some of the conidia were not in direct contact with the cuticle but were arranged in small and large aggregates. These conidia may have been dislodged from the tick surface and released into the surrounding area over the course of the 24-h period of the experiment.
A correlation between the number of adhered conidia and host mortality has been observed for M. anisopliae on thrips cuticle (18). In our study, the adhesion of M. anisopliae var. anisopliae strain 7 conidia to tick cuticle was dose dependent and was also strongly correlated with host mortality. Interestingly, when ticks were inoculated with a fungal suspension of 1 × 105 or 1 × 106 conidia per ml, a profound increase in tick mortality was observed from <20% to 60%, respectively, even though the number of adhered conidia increased by only 2.6. This observation may suggest the existence of an adhesion threshold necessary for causing high mortality. Moreover, despite an increase in the absolute real number of conidia that adhered to each cuticle, the relative percentage of adhered conidia out of total conidia applied was sharply reduced as the concentration of conidia in the inoculum suspension increased (Table 2). One possible explanation for this phenomenon may be that as the concentration of conidia increases, there are more hydrophobic interactions between individual conidia, so that the conidia attach to each other rather than to the tick cuticle. These aggregations lead to a decrease in the number of free conidia that can actually adhere to the host cuticle. This phenomenon may reduce the efficacy of fungal formulation. One can speculate that by using a higher concentration of surface active agents or an oil-based formulation, we may be able to limit the aggregation of the conidia so that a higher percentage of the conidia attach to the hosts.
TABLE 2.
Influence of concentration of M. anisopliae var. anisopliae strain 7 conidia in inoculum on numbers of conidia adhering to the cuticle of an engorged R. annulatus female
| Log of concn of conidia in inoculum | No. of conidia per tick in inoculuma | Avg no. of conidia adhering to tickb | % of applied conidia adhering to tick |
|---|---|---|---|
| 4 | 20,000 | 5,500 ± 2,739 | 27.50 |
| 5 | 200,000 | 9,029 ± 2,526 | 4.51 |
| 6 | 2,000,000 | 23,800 ± 6,614 | 1.19 |
| 7 | 20,000,000 | 111,458 ± 9,361 | 0.56 |
| 8 | 200,000,000 | 262,000 ± 24,070 | 0.13 |
Five ticks were individually dipped (for 5 s) into 2-ml conidial suspensions of the corresponding concentrations.
Data are also shown in Fig. 3.
The hydrophobic conidia of entomopathogenic fungi bind in a nonspecific manner to the epicuticular surfaces of both susceptible and resistant hosts (6, 15). However, host specificity at the attachment stage has rarely been demonstrated (27, 43). A very specific case of decreasing conidial adhesion corresponding to the presence of specific cuticular compounds was described by Lord and Howard (25) for the insect Liposcelis bostrychophila (Psocoptera: Liposcelidae), which is highly resistant to entomopathogenic fungi. A fatty amide present in this insect's cuticle contributes to its tolerance of fungi by decreasing the hydrophobicity of its cuticle (25). The fact that the adhesion of M. anisopliae var. anisopliae strain 7 conidia to the tick cuticle is a nonspecific process was demonstrated in this study. The conidia adhered to cuticles of both susceptible and resistant hosts, and the number of conidia adhering per cm2 of cuticle was not related to the degree of resistance of the tick species to the pathogen. This method was also found to be applicable for A. diaperinus larvae and for conidia of M. anisopliae var. acridum strain 5, indicating that it can be used to study other arthropods and other species of fungi.
The DCM extraction method removed both ungerminated adhered conidia and conidia that had already germinated on the cuticle, but not conidia that had produced appressoria. Thus, the suggested method can easily show the amount of adhered conidia as well as the conidial germination dynamic on host. The appressoria of M. anisopliae are known to secrete a layer of mucilaginous material (6, 24, 39) that increases the adhesive capability of the pathogen. The conidia that have germinated and formed appressoria are tightly attached to deeper layers of the cuticle and cannot be removed by enzymatic treatments (20). Our SEM observations indicated also that, after 24 h of exposure to tick cuticle, some germinated conidia could not be removed from the cuticle using DCM. Usually, these remaining conidia were firmly attached to the tick cuticle by appressoria. M. anisopliae hydrophobin genes are highly expressed during the formation of appressoria (40, 41) and as has been proposed by Wosten and Wessels (47), this phenomenon can promote firmer attachment of the appressorium to the host surface before penetration.
Although most of the experiments were carried out using two tick species and one strain of M. anisopliae, this method may be adapted to other arthropod-fungus pairs. The described method can be used to study the different aspects of early host-pathogen interactions and the persistence of the conidia on the host cuticle as well as for evaluating the distribution efficacy of the fungi propagules under diverse environmental and application conditions. Also, adhesion of different mycoinsecticide formulations to host cuticle can be compared using this method. Understanding these properties may lead to an intelligent use of these entomopathogens in pest control and support the development of mycoinsecticides.
Acknowledgments
We are grateful to Naomi Bahat (Interdepartmental Equipment Unit, Faculty of Agriculture, Food and Environment of the Hebrew University, Rehovot, Israel) for preparing ticks for SEM and to Benni Ginzburg for adapting the photographs for print.
This work was supported by the Environment, Science, and Technology Bureau for Economic Growth, Agriculture and Trade, U.S. Agency for International Development, under the terms of award no. TA-MOU-03-C22-008.
Footnotes
Published ahead of print on 2 April 2010.
REFERENCES
- 1.Alston, D. G., D. E. N. Rangel, L. A. Lacey, H. G. Golez, J. J. Kima, and D. W. Roberts. 2005. Evaluation of novel fungal and nematode isolates for control of Conotrachelus nenuphar (Coleoptera: Curculionidae) larvae. Biol. Control 35:163-171. [Google Scholar]
- 2.Altre, J. A., J. D. Vandenberg, and F. A. Cantone. 1999. Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J. Invertebr. Pathol. 73:332-338. [DOI] [PubMed] [Google Scholar]
- 3.Arruda, W., I. Lubeck, A. Schrank, and M. H. Vainstein. 2005. Morphological alterations of Metarhizium anisopliae during penetration of Boophilus microplus ticks. Exp. Appl. Acarol. 37:231-244. [DOI] [PubMed] [Google Scholar]
- 4.Askary, H., N. Benhamou, and J. Brodeur. 1999. Ultrastructural and cytochemical characterization of aphid invasion by the hyphomycete Verticillium lecanii. J. Invertebr. Pathol. 74:1-13. [DOI] [PubMed] [Google Scholar]
- 5.Bidochka, M. J., R. J. St. Leger, and D. W. Roberts. 1997. Mechanisms of Deuteromycete fungal infections in grasshoppers and locusts: an overview. Mem. Entomol. Soc. Can. 171:213-224. [Google Scholar]
- 6.Boucias, D., and J. Pendland. 1991. Attachment of mycopathogens to cuticle, p. 101-127. In G. T. Cole and H. C. Hoch (ed.), The fungal spore and disease initiation in plants and animals. Plenum Press, New York, NY.
- 7.Boucias, D. G., J. C. Pendland, and J. P. Latge. 1988. Nonspecific factors involved in attachment of entomopathogenic deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 54:1795-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, A., and R. Wall. 2005. Horizontal transmission of fungal infection by Metarhizium anisopliae in parasitic Psoroptes mites (Acari: Psoroptidae). Biol. Control 34:58-65. [Google Scholar]
- 9.Butt, T. M., L. Ibrahim, S. J. Clark, and A. Beckett. 1995. The germination behavior of Metarhizium anisopliae. Mycol. Res. 99:945-950. [Google Scholar]
- 10.Fargues, J. 1984. Adhesion of the fungal spore to the insect cuticle in relation to pathogenicity, p. 90-110. In D. W. Roberts and J. R. Aist (ed.), Infection processes of fungi. Rockefeller Foundation Conference Reports, New York, NY.
- 11.Feng, M. G., and J. H. Xu. 2002. Attachment and invasion of Pandora delphacis conidia into the integument of the green peach aphid, Myzus persicae. Mycosystema 21:270-273. [Google Scholar]
- 12.Gilby, R. A. 1957. Studies of cuticular lipids of arthropods. III. The chemical composition of the wax from Boophilus microplus. Arch. Biochem. Biophys. 67:320-324. [DOI] [PubMed] [Google Scholar]
- 13.Gindin, G., M. Samish, E. Alekseev, and I. Glazer. 2001. The susceptibility of Boophilus annulatus (Ixodidae) ticks to entomopathogenic fungi. Biocontrol Sci. Technol. 11:113-120. [Google Scholar]
- 14.Gindin, G., M. Samish, G. Zangi, A. Mishoutchenko, and I. Glazer. 2002. The susceptibility of different species and stages of ticks to entomopathogenic fungi. Exp. Appl. Acarol. 28:283-288. [DOI] [PubMed] [Google Scholar]
- 15.Hajek, A. E., and C. C. Eastburn. 2003. Attachment and germination of Entomophaga maimaiga conidia on host and non-host larval cuticle. J. Invertebr. Pathol. 82:12-22. [DOI] [PubMed] [Google Scholar]
- 16.Holder, D. J., and N. O. Keyhani. 2005. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl. Environ. Microbiol. 71:5260-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim, L., T. M. Butt, and P. Jenkinson. 2002. Effect of artificial culture media on germination, growth, virulence and surface properties of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol. Res. 106:705-715. [Google Scholar]
- 18.Iwase, R., and S. Shimizu. 2004. Adhesion and virulence of conidia of Metarhizium anisopliae to Thrips palmi (Thysanoptera: Thripidae). Jpn. J. Appl. Entomol. Zool. 48:275-280. [Google Scholar]
- 19.James, R. R., J. S. Buckner, and T. P. Freeman. 2003. Cuticular lipids and silverleaf whitefly stage affect conidial germination of Beauveria bassiana and Paecilomyces fumosoroseus. J. Invertebr. Pathol. 84:67-74. [DOI] [PubMed] [Google Scholar]
- 20.Jarrold, S. L., D. Moore, U. Potter, and A. K. Charnley. 2007. The contribution of surface waxes to pre-penetration growth of an entomopathogenic fungus on host cuticle. Mycol. Res. 111:240-249. [DOI] [PubMed] [Google Scholar]
- 21.Kirkland, B. H., E. M. Cho, and N. O. Keyhani. 2004. Differential susceptibility of Amblyomma maculatum and Amblyomma americanum (Acari: Ixodidae) to the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. Biol. Control 31:414-421. [Google Scholar]
- 22.Kirkland, B. H., G. S. Westwood, and N. O. Keyhani. 2004. Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J. Med. Entomol. 41:705-711. [DOI] [PubMed] [Google Scholar]
- 23.Lacey, C. M., L. A. Lacey, and D. R. Roberts. 1988. Route of invasion and histopathology of Metarhizium anisopliae in Culex quinquefasciatus. J. Invertebr. Pathol. 52:108-118. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Llorca, L. V., C. Olivares-Bernabeu, J. Salinas, H. B. Jansson, and P. E. Kolattukudy. 2002. Pre-penetration events in fungal parasitism of nematode eggs. Mycol. Res. 106:499-506. [Google Scholar]
- 25.Lord, J. C., and R. W. Howard. 2004. A proposed role for the cuticular fatty amides of Liposcelis bostrychophila (Psocoptera: Liposcelidae) in preventing adhesion of entomopathogenic fungi with dry conidia. Mycopathologia 158:211-217. [DOI] [PubMed] [Google Scholar]
- 26.McCauley, V. J. E., R. Y. Zacharuk, and R. D. Tinline. 1968. Histopathology of green muscardine in larvae of four species of Elateridae (Coleoptera). J. Invertebr. Pathol. 12:444-459. [Google Scholar]
- 27.McGuire, M. R. 1985. Erynia radicans: studies on its distribution, pathogenicity, and host range in relation to potato leafhopper, Empoasca fabae. Ph.D. thesis. University of Illinois, Urbana, IL.
- 28.Meyer, U., and H. Sermann. 2003. Fluorescence microscopic investigations on adhesion of spores of the entomopathogenic fungus Verticillium lecanii at larvae of Frankliniella occidentalis (Thysanoptera: Thripidae). Bull. OILB/SROP 26:125-128. [Google Scholar]
- 29.Meyer, U., H. Sermann, and C. Buettner. 2002. Spore adhesion of entomopathogenic fungi to larvae of Frankliniella occidentalis (Pergande, 1895) (Thysanoptera: Thripidae). Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 67:601-607. [PubMed] [Google Scholar]
- 30.Moino, A., Jr., S. B. Alves, R. B. Lopes, P. M. O. J. Neves, R. M. Pereira, and S. A. Vieira. 2002. External development of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in the subterranean termite Heterotermes tenius. Sci. Agric. 59:267-273. [Google Scholar]
- 31.Neves, P. M. O. J., and S. B. Alves. 2000. Grooming capacity inhibition in Cornitermes cumulans (Kollar) (Isoptera: Termitidae) inoculated with entomopathogenic fungi and treated with imidacloprid. Ann. Soc. Entomol. Bras. 29:537-545. [Google Scholar]
- 32.O'Conell, J. P., and J. M. Haile. 2005. Thermodynamics—fundamentals for application, p. 582. Cambridge University Press, Cambridge, United Kingdom.
- 33.Quintela, E. D., and C. W. McCoy. 1998. Conidial attachment of Metarhizium anisopliae and Beauveria bassiana to the larval cuticle of Diaprepes abbreviatus (Coleoptera: Curculionidae) treated with imidacloprid. J. Invertebr. Pathol. 72:220-230. [DOI] [PubMed] [Google Scholar]
- 34.Raghuvanshi, R. S., S. Goyal, O. Singh, and A. K. Panda. 1998. Stabilization of dichloromethane-induced protein denaturation during microencapsulation. Pharm. Dev. Technol. 3:269-276. [DOI] [PubMed] [Google Scholar]
- 35.Samish, M., H. Ginsberg, and I. Glazer. 2008. Anti-tick biological control agents: assessment and future perspectives, p. 447-469. In A. S. Bowman and A. Nuttall (ed.), Tick biology, disease and control. Cambridge University Press, Cambridge, United Kingdom.
- 36.Samuels, K. D. Z., D. E. Pinnock, and P. G. Allsopp. 1989. The potential of Metarhizium anisopliae (Metschnikoff) Sorokin (Deutermycotina, Hyphomycetes) as a biological control agent of Inopus rubriceps (Macquart) (Diptera, Stratiomyidae). J. Aust. Entomol. Soc. 28:69-74. [Google Scholar]
- 37.Sitch, J. C., and C. W. Jackson. 1997. Pre-penetration events affecting host specificity of Verticillium lecanii. Mycol. Res. 101:535-541. [Google Scholar]
- 38.Sosa-Gomez, D. R., D. G. Boucias, and J. L. Nation. 1997. Attachment of Metarhizium anisopliae to the Southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. J. Invertebr. Pathol. 69:31-39. [DOI] [PubMed] [Google Scholar]
- 39.St. Leger, R. J., T. M. Butt, M. S. Goettel, R. C. Staples, and D. W. Roberts. 1989. Production of appressoria by the entomopathogenic fungus Metarhizium anisopliae. Exp. Appl. Mycol. 13:274-288. [Google Scholar]
- 40.St. Leger, R. J., R. C. Staples, and D. W. Roberts. 1992. Cloning and regulatory analysis of starvation-stress gene, ssgA, encoding a hydrophobin-like protein from the entomopathogenic fungus, Metarhizium anisopliae. Gene 120:119-124. [DOI] [PubMed] [Google Scholar]
- 41.Talbot, N. J., D. J. Ebbole, and J. E. Hamer. 1993. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5:1575-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestergaard, S., T. M. Butt, J. Bresciani, A. T. Gillespie, and J. Eilenberg. 1999. Light and electron microscopy studies of the infection of the Western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae) by the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 73:25-33. [DOI] [PubMed] [Google Scholar]
- 43.Vey, A., J. Fargues, and P. Robert. 1982. Histological and ultrastructural studies of factors determining the specificity of pathotypes of the fungus Metarhizium anisopliae for scarabaeid larvae. Entomophaga 27:387-397. [Google Scholar]
- 44.Wang, C., and R. J. St. Leger. 2007. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 6:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wessels, J. G. H. 2000. Hydrophobins, unique fungal proteins. Mycologist 14:153-159. [Google Scholar]
- 46.Wösten, H. A. B. 2001. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 55:625-646. [DOI] [PubMed] [Google Scholar]
- 47.Wösten, H. A. B., and J. G. H. Wessels. 1997. Hydrophobins, from molecular structure to multiple functions in fungal development. Mycoscience 38:363-374. [Google Scholar]
- 48.Yaginuma, D., H. Hiromori, and M. Hatsukade. 2004. Relationship between virulence and adhesion of the entomopathogenic fungus Beauveria amorpha (strain: HpBa-1) to the yellowish elongate chafer, Heptophylla picea (Motschulsky) (Coleoptera: Scarabaeidae). Jpn. J. Appl. Entomol. Zool. 48:101-108. [Google Scholar]