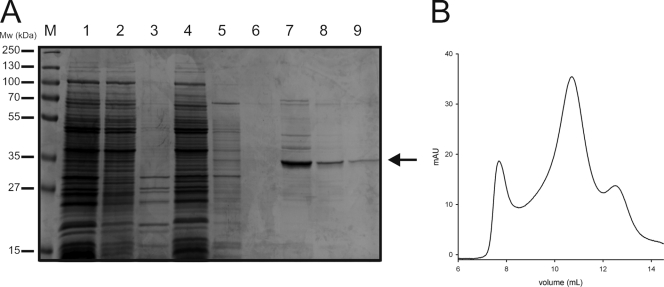
FIG. 1.
Purification of DctABs. (A) Protein samples from different steps throughout the purification were run on an SDS-12.5% polyacrylamide gel, which was stained with Coomassie brilliant blue. Lanes: 1, membrane vesicles (10 μg of protein); 2, DDM-soluble fraction; 3, insoluble fraction; 4, flowthrough from the nickel-Sepharose column; 5, wash fraction from the Ni-Sepharose column; 6 to 8, three elution fractions from the nickel-Sepharose column; and 9, peak fraction (at an elution volume of ∼11 ml) from the size exclusion chromatography step. The arrow indicates DctABs. M, markers; Mw, molecular mass. (B) The peak elution fraction from the nickel-Sepharose column (lane 7) was subjected to size exclusion chromatography with a Superdex-200 column. The chromatogram is shown.