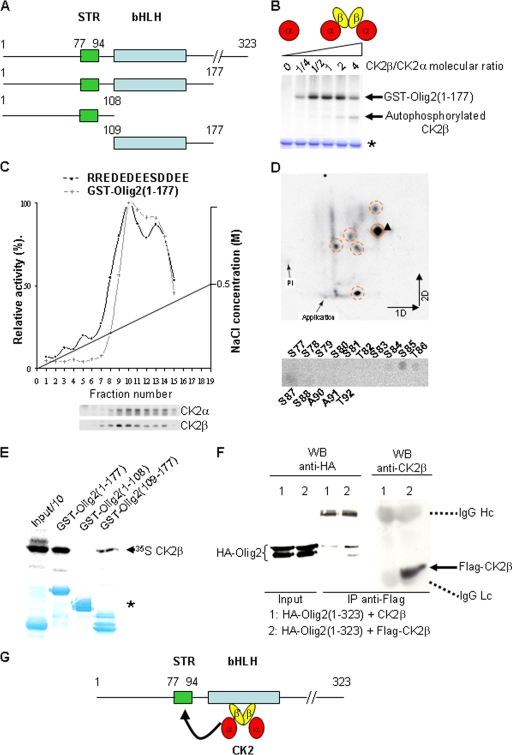
FIG. 8.
CK2β positively modulates CK2-dependent phosphorylation of the Olig2(1-177) fragment in vitro. (A) Primary structure of mouse Olig2 and schematic representation of the Olig2 fragments used in this study. Represented are the serine-threonine-rich domain sequence (STR, amino acids 77 to 94), and the basic helix-loop-helix sequence (bHLH, amino acids 109 to 164). (B) Autoradiography of the purified recombinant GST-Olig2(1-177) fusion fragment after in vitro phosphorylation by the CK2 catalytic subunit (α) alone or in combination with increasing amounts of the CK2 regulatory subunit (β), which generate increased amounts of the holoenzyme (α2β2). Note that CK2β, an autosubstrate of the catalytic α subunit, competed in the reaction for the CK2 phosphorylation of Olig2 when present in excess. Protein normalization was controlled by Coomassie blue staining (*). (C) DEAE-Sephacel chromatography of crude wild-type E18.5 forebrain protein extracts. Each fraction was analyzed for consensus relative CK2 activity with the RRREDEESDDEE synthetic substrate/peptide and for relative CK2β-dependent Olig2(1-177) kinase activity. Activity profiles were normalized to the activity detected in the peak fractions. In parallel, a sample of each fraction was immunoblotted to detect the presence of CK2α and CK2β proteins (lower panels). (D) Two-dimensional (2D) phosphopeptide mapping analysis of CK2β-dependent Olig2(1-177) phosphorylation. The major phosphopeptide (arrowhead; upper panel) was subjected to automated Edman degradation and released derivates from each cycle were spotted onto plates and autoradiographed (lower panel). Deduced amino acids in the Olig2 sequence are indicated above or below the spots. (E) Recombinant GST fusion proteins containing different Olig2 fragments (1 to 177, 1 to 108, and 109 to 177) were incubated with [35S]methionine-labeled CK2β. The ability of the GST fusion proteins to pull down the CK2β subunit was detected by autoradiography after electrophoresis. On the left, one-tenth of the input is shown. Loading controls were checked after Coomassie blue staining of the gel (*). (F) Coimmunoprecipitation of Flag-CK2β and HA-Olig2(1-323) in Cos7 cells. Flag-CK2β was immunoprecipitated (IP) with anti-Flag antibody, and the immunoprecipitated complexes were analyzed by Western blotting (WB) with anti-HA and anti-CK2β antibodies. Hc, heavy chain; Lc, light chain. (G) Schematic representation of the suggested CK2-Olig2 interaction.