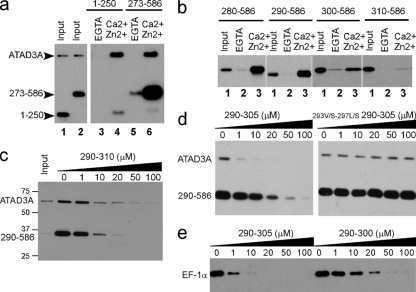
FIG. 3.
Characterization of the S100B binding domain of ATAD3A. (a and b) Pulldown mapping of the S100B binding site on ATAD3A. (a) ATAD3A fragments comprising residues 1 to 250 and 273 to 586 are expressed in U373 cells. The respective cell lysates (lanes 1 and2) were tested by S100B pulldown with EGTA (lanes 3 and 5)- or Ca2+ and Zn2+ (lanes 4 and 6)-containing buffer. (b) The fragments comprising residues 280 to 586, 290 to 586, 300 to 586, and 310 to 586 of ATAD3A were tested according to the same process described above (a). Lane 1, input; lane 2, EGTA; lane 3, Ca2+ and Zn2+. Endogenous ATAD3A (a) and its fragments (a and b) were revealed by Western blotting using a mixture of ATAD3A N-ter and C-ter antibodies. (c to e) Characterization of the minimal S100B binding domain on ATAD3A. (c) ATAD3A290-310 functions as a competitor for the Ca2+-dependent interaction of ATAD3A and the fragment at residues 290 to 586 with S100B-Sepharose beads. (d) ATAD3A290-305 but not its mutant counterpart 293V/S-297L/S-290-305 functions as a competitor for the Ca2+-dependent interaction of ATAD3A and the fragment at residues 290 to 586 with S100B-Sepharose beads. (e) Comparison of ATAD3A peptide fragments at residues 290 to 305 and 290 to 300 as competitors for the Ca2+-dependent interaction of EF-1α with S100B-Sepharose beads.U373 cell lysates were tested by S100B pulldown with Ca2+- and Zn2+-containing buffer in the presence of increasing concentrations of peptide competitor P290-305, as indicated. Endogenous ATAD3A and its fragment at residues 290 to 586 (c and d) or EF-1α (e) were revealed by Western blotting.