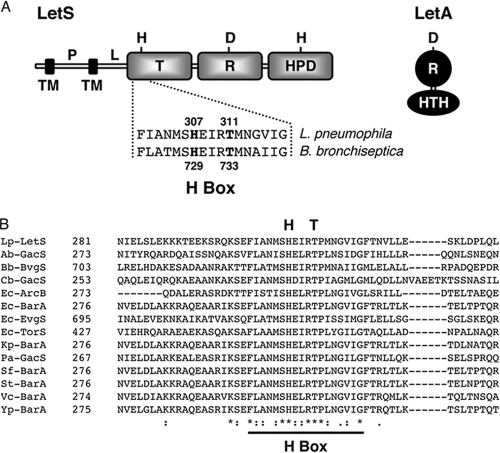
FIG. 1.
The L. pneumophila LetA/LetS two-component system. (A) Domain architecture of the LetA/LetS two-component system. LetS, a 103-kDa sensor protein, is likely tethered to the inner membrane by two transmembrane (TM) domains at its N terminus. The periplasmic (P) domain is connected via a linker (L) region to three cytoplasmic signaling domains, a transmitter (T), receiver (R), and histidine phosphotransfer domain (HPD). LetA is a 43-kDa activator kinase that contains a receiver (R) domain and a helix-turn-helix motif (HTH). It is predicted that, upon receiving a signal, LetS autophosphorylates on a conserved histidine residue, and then the phosphate is sequentially transferred to aspartic acid and histidine residues in LetS and finally to an aspartic acid in LetA. A histidine-to-glutamine substitution at amino acid 307 of LetS abolishes LetS activity, while a threonine-to-methionine substitution at position 311 creates a strain with sluggish transcriptional and phenotypic profiles. (B) Sequence alignment of the L. pneumophila LetS H-box region with related sensor kinases. Amino acid alignments were produced using T-Coffee. Dashes represent gaps introduced to optimize sequence alignments. Asterisks indicate identical residues while the colon and period represent conserved and semiconserved amino acids, respectively. The H-box region is underlined, and the primary histidine and conserved threonine residues are displayed above the alignment. Lp, L. pneumophila; Ab, A. baumannii; Bb, B. bronchiseptica; Cb, C. burnetii; Ec, E. coli; Kp, K. pneumoniae; Pa, P. aeruginosa; Sf, S. flexneri; St, S. Typhimurium; Vc, V. cholerae; Yp, Y. pestis.