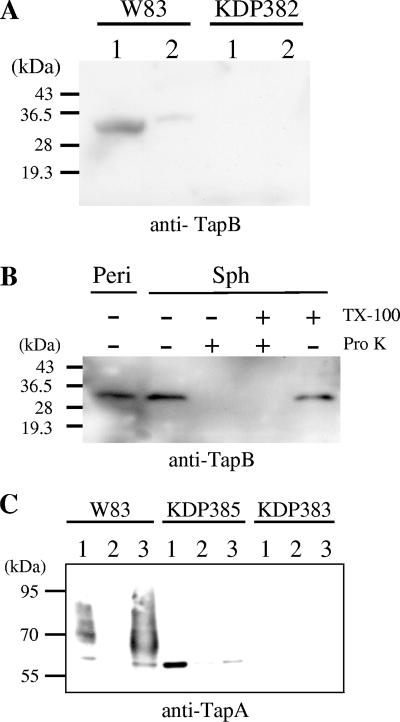
FIG. 5.
Localization of TapA and TapB proteins. (A and B) Subcellular localization of TapB protein. (A) Fractions of cytoplasm-periplasm (lanes 1) and total membrane (lanes 2) of W83 (wild type) and KDP382 (ΔtapB::ermF) were subjected to SDS-PAGE and immunoblot analysis using anti-TapB. (B) Spheroplast and periplasm fractions of P. gingivalis cells were separated as described in Materials and Methods. Spheroplasts were subjected to the proteinase K treatment (Pro K) in the presence or absence of 2% Triton X-100 (TX-100). Samples were subjected to SDS-PAGE followed by immunoblot analysis with anti-TapB. Sph, spheroplasts; Peri, periplasm fraction. (C) Subcellular localization of the TapA protein. Cytoplasm-periplasm, inner membrane, and outer membrane fractions of W83 (wild type), KDP385 (ΔporT::erm), and KDP383 (ΔtapA::ermF) were subjected to SDS-PAGE and immunoblot analysis using anti-TapA. Lanes: 1, cytoplasm-periplasm fraction; 2, inner membrane fraction; 3, outer membrane fraction.