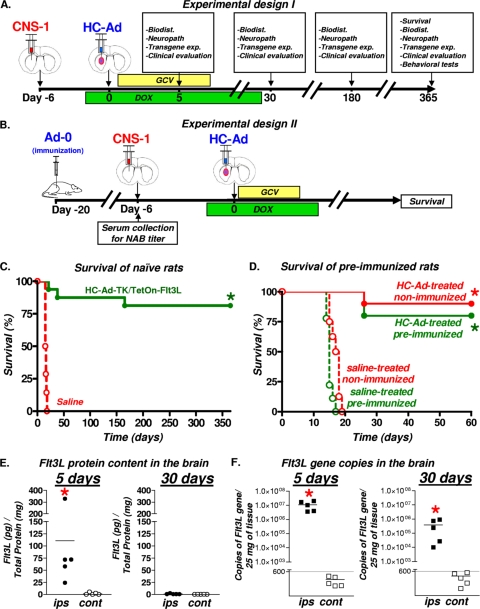
FIG. 2.
Efficacy and Flt3L expression after intratumoral administration of therapeutic bicistronic HC-Ad. (A) HC-Ad-TK/TetOn-Flt3L (16/group) or saline (14/group) was delivered intratumorally into 6-day intracranial CNS1 tumors. (B) For preimmunization studies, animals were preimmunized with either saline or Ad-0. Two weeks later, animals were implanted with CNS1 tumor cells and then treated 6 days later with an intratumoral injection of either saline or HC-Ad (8 to 10/group). (C) Kaplan-Meier survival curves of naïve tumor-bearing rats treated with bicistronic therapeutic HC-Ad. *, P < 0.05 versus saline (log-rank test). (D) Kaplan-Meier survival curves of preimmunized animals treated with bicistronic therapeutic HC-Ad. *, P < 0.05 versus saline (log-rank test). (E) Levels of Flt3L protein in brain hemispheres ipsilateral (ips) and contralateral (cont) to the bicistronic HC-Ad injection site were assessed by ELISA at 5 and 30 days after treatment (5/group). *, P < 0.05 versus the contralateral value (Student's t test). (F) DNA was isolated from both brain hemispheres (5/group), and Flt3L transgene copies were quantified by qPCR at 5 and 30 days after delivery of bicistronic HC-Ad. *, P < 0.05 versus the contralateral value (Student's t test).