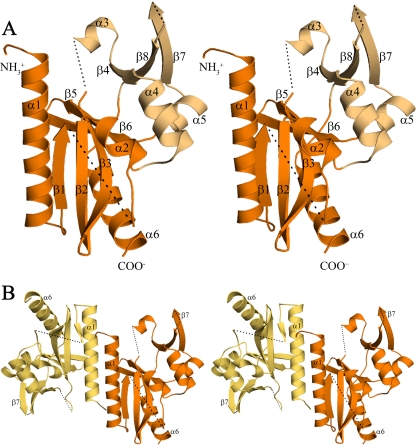
FIG. 1.
The structure of D212 (stereo images). (A) The D212 protomer (chain B) adopts the restriction endonuclease fold. The central “catalytic” domain (orange) is composed of a mixed, five-stranded β-sheet (β1, β2, β3, β5, and β6) packed between α-helices (α1/α6 and α2). The smaller “DNA recognition” domain (light orange) is composed of an antiparallel three-stranded β sheet (β4, β7, and β8) along with three α-helices, α3, α4, and α5. Dashed lines represent chain breaks. (B) The D212 dimer oriented as in panel A, with chain A in yellow-orange and chain B in orange. The dimer interface is formed by residues at the N terminus, the α1 helix, and strand β1. It is primarily hydrophobic and buries ∼12% of the subunit surface area.