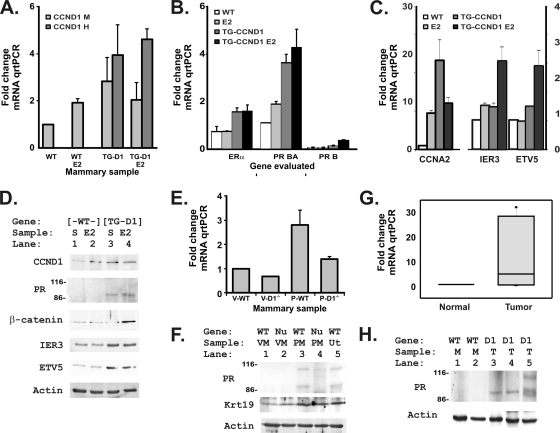
FIG. 2.
Estradiol increases progesterone receptor expression in mammary tissues of mice constitutively expressing cyclin D1. (A) Levels of both murine CCND1 mRNA (CCND1 M) and the human CCND1 mRNA that was used in our transgene (CCND1 H) were assessed by TaqMan-based quantitative real-time PCRs (qRT-PCR) to evaluate cDNAs reverse transcribed from 5 μg of total RNA harvested from each of the indicated specimens 2 months after hormonal treatments. We measured mRNA and cDNA amounts at each step in the analyses and used equal amounts of mRNA and cDNAs for each tissue and gene evaluated for each qRT-PCR. Murine cyclin D1 (CCND1 M) and human cyclin D1 (CCND1 H) levels were determined and plotted as fold changes in the threshold value for quantitation, setting the untreated wild-type (WT) mammary sample to a value of 1 and plotting fold changes for each of the other samples. The results for wild-type (WT), E2-treated wild-type (WT E2), CCND1 transgenic (TG-D1), and E2-treated CCND1 transgenic (TG-D1 E2) mice are indicated. The means and standard errors for 3 samples are shown. Human CCND1 was undetectable in wild-type mice, and the murine CCND1 primer-probe set did not detect human CCND1. (B) Estrogen receptor (ER) and progesterone receptor (PR) mRNAs were evaluated with TaqMan as described for panel A, using the specific primers and probes listed in Materials and Methods. The PR BA mRNA is 300 nucleotides longer than the PR A transcript. PR B isoform-specific primers were used to differentiate expression of this additional 5′ sequence (34). (C) Changes in cyclin A2 (CCNA2) expression were evaluated in the same tissues to detect possible CDK- and E2F-dependent effects. Increases for immediate-early response 3 (IER3) and ets variant 5 (ETV5), two candidate CCND1-target mRNAs, were evaluated in the same tissues. (D) Mammary protein lysates (50 μg) were probed for CCND1 (by using an antibody that detects both human and mouse cyclin D1), the progesterone receptor (PR), β-catenin, and the two candidate CCND1-target genes for which antibodies could detect the murine protein in mammary tissues. Size markers show that numbers of the PR A isoform are increased in the cyclin D1 transgenic mice, consistent with reports that PR A is the dominant PR isoform except during the later stages of pregnancy (1). An actin loading control is included. Wild-type (WT) and cyclin D1 transgenic (TG-D1) genotypes are indicated. Treatment groups were subjected to sham treatment (S) or treated with estradiol pellets (E2). (E) Progesterone receptor mRNA levels were measured in mammary tissues from virgin (V) and 12-day-pregnant (P), wild-type (WT), and CCND1−/− (D1−/−) mice. The mRNAs were isolated and measured as described above for panel A. Values are plotted as means and standard deviations for two glands of each developmental stage and phenotype. (F) Protein lysates from mammary glands of virgin (VM), 12-day-pregnant (PM), wild-type (WT), and CCND1−/− (Nu) mice were probed and compared with those of a wild-type uterine sample (Ut). In this case, both 114-kDa and 94-kDa PR bands are seen for the pregnant tissues, consistent with the reported expression of PR B and A during pregnancy (1). The membrane was then probed for keratin 18, a luminal mammary cell marker (2), to show that the PR changes were not due simply to changes in tissue composition. An actin loading control is shown. (G) Progesterone receptor mRNA was measured in mammary lysates from normal mammary tissues (Normal) and tumors induced by the MMTV-cyclin D1 transgene (Tumor). The mRNAs were isolated and measured as described above for panel A. (H) Lysates harvested from normal mammary glands (M) and cyclin D1-transgene-induced tumors (T) were probed for PR protein and an actin loading control.