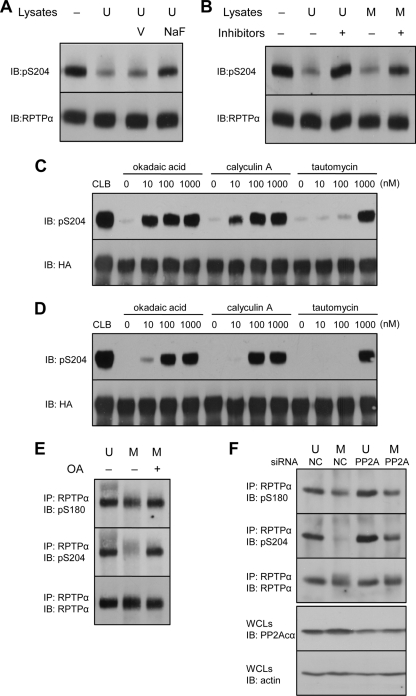
FIG. 5.
PP2A dephosphorylates RPTPα in vitro and in vivo. (A to D) Endogenous RPTPα was immunoprecipitated from unsynchronized NIH 3T3 cells (A and B). The immunoprecipitates were incubated with fresh lysates of unsynchronized (U) or mitotic (M) NIH 3T3 cells in the presence of orthovanadate (V), sodium fluoride (NaF), or a cocktail of PP1/PP2A inhibitors containing okadaic acid, calyculin A, and tautomycin (100 nM each). After the reactions were terminated, the proteins were separated by 7.5% SDS-PAGE and transferred to PVDF membranes. The blots were probed with anti-pS204 antibody and subsequently, after stripping, with anti-RPTPα antibody. HA-RPTPα overexpressed in COS1 cells was immunoprecipitated and incubated with unsynchronized (C) or mitotic (D) NIH 3T3 lysates in the presence of increasing amounts of the PP1/PP2A inhibitors as indicated. As a control, immunoprecipitated HA-RPTPα was incubated with cell lysis buffer (CLB). The lysates were removed, and the samples were processed as for panel A. Finally, the blots were probed with anti-pS204 antibody and subsequently, after stripping, with anti-HA tag antibody. (E) NIH 3T3 cells arrested with nocodazole were treated with 100 nM okadaic acid (OA). RPTPα was immunoprecipitated and blotted, and the blots were probed with pS180, pS204, and RPTPα antibodies. These experiments have been done at least three times, and representative blots are shown. (F) siRNA-mediated knockdown of PP2Acα in unsynchronized (U) or mitotic (M) NIH 3T3 cells was done as described in Materials and Methods. Efficiency of knockdown was monitored by blotting using a PP2Acα-specific antibody and equal loading using an actin antibody (bottom two panels). RPTPα was immunoprecipitated, blotted, and probed using pS180, pS204, and RPTPα antibodies, as indicated. IP, immunoprecipitation; IB, immunoblot.