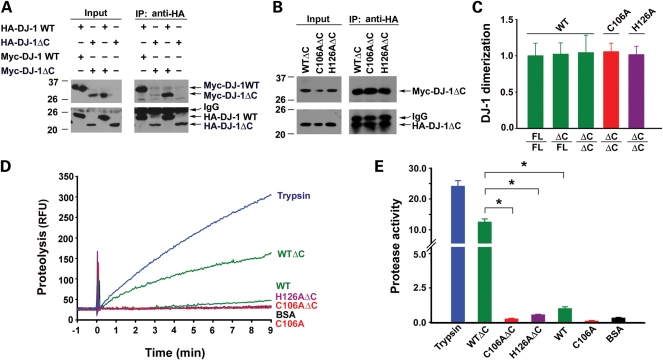
Figure 2.
Deletion of the C-terminal tail enhances DJ-1 protease activity without affecting its dimerization. (A and B) Lysates from HeLa cells expressing the indicated HA- and/or Myc-tagged DJ-1 proteins were immunoprecipitated with anti-HA antibody, followed by immunoblotting with anti-Myc and anti-HA antibodies. (C) DJ-1 dimerization was determined by quantification of the intensity of co-immunoprecipitated Myc-tagged DJ-1 band and normalized to the amount of HA-tagged DJ-1 band in the same immunoprecipitate. Data represents mean ± SEM from three independent experiments. (D) Protease activity of the indicated proteins was measured by a continuous, real-time fluorescence-based protease assay using BODIPY FL-casein (10 µg/ml) as the substrate. Fluorimetric recordings of 0.6 µm trypsin (blue), 50 µm DJ-1 WT or DJ-1 WTΔC (green), DJ-1 C106A or DJ-1 C106AΔC (red), DJ-1 H126AΔC (purple) and BSA (black). RFU, relative fluorescence units. (E) Protease activity of each protein was quantified by measuring the initial velocity of the proteolytic reaction from the fluorimetric trace shown in Fig. 2D. Protease activity on the Y-axis indicates the measured protease activity of each protein after normalization to the protease activity of DJ-1 WT. Data represents mean ± SEM from three independent experiments. *P < 0.05.