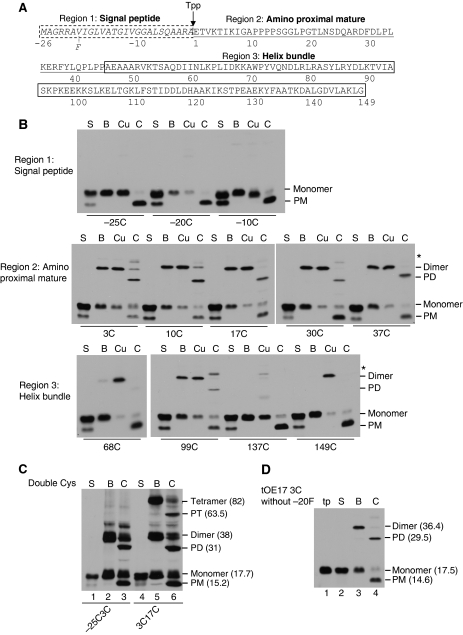
Figure 2.
Precursors oligomerize on the thylakoid membrane primarily through Cys residues near the amino-terminus of the mature domain. (A) Diagram of the maize tOE17 precursor protein sequence. Amino acids are numbered relative to the thylakoid processing signal peptidase (Tpp) cleavage site. The signal peptide and two structural domains of the mature protein (Balsera et al, 2005; Ristvejova et al, 2006) are depicted. Precursor proteins in (B) and (C) also contained a phenylalanine (F) in position −20 (Gerard and Cline, 2007). (B) Binding and chase assays with precursors substituted with unique Cys residues at positions designated in the figure. Membranes recovered from binding assays were washed with IB (lane B), treated with the oxidant CuP (lane Cu), or subjected to a chase assay (lane C). Lane S contains supernatant from the binding assay. Samples were analysed by non-reducing SDS–PAGE and fluorography. (C) Binding and chase assays with the double Cys precursor [3H] tOE17-25C-20F3C and precursor [3H] tOE17-20F3C17C. Designations of protein species are as in Figure 1 except that putative precursor tetramers and the processed tetramers (PT) are indicated. Their average Mrs (in kilodaltons) are indicated. (D) Binding and chase assays with [3H] tOE173C, which has the wild-type sequence in the hydrophobic core of the signal peptide.