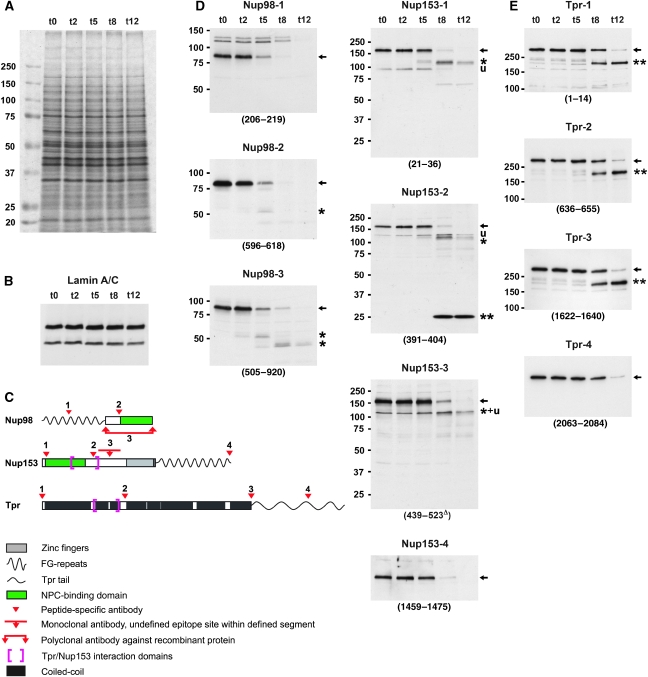
Figure 3.
Nup153 and Nup98 appear largely degraded upon PV infection whereas the coiled-coil rod domain of protein Tpr remains intact. (A) SDS–PAGE and Coomassie staining of whole-protein extracts from HeLa cells before and at 2–12 h post-infection, showing the bulk of cellular proteins unaffected by PV-induced proteolysis. The extracts were from the same HeLa cell cultures analysed by TEM in Figure 1. (B) Immunoblotting of extracts used in panel A, showing that lamin A and lamin C, like lamin B (not shown), remain unaffected. This indicates that rearrangements within the nuclear lamina or its NE attachments, but not lamin proteolysis, are required for the NE upfolding observed. (C) Epitope sites of Nup153, Nup98, and Tpr antibodies indicated by arrowheads. Different antibodies targeting the same protein are numbered as in panels D and E. (D, E) Immunoblotting of Nup153, Nup98, and Tpr, using cell extracts shown in panel A; (see also Supplementary Figure S3). Target regions are given in parentheses; Δ indicates mAbs for which actual epitopes within defined protein segments are unknown. (D) At 8–12 h post-infection, when HEZs are visible at almost all NPCs, Nup153 (full-length proteins marked by arrows) appears largely degraded, except for a small segment (double-asterisk) comprising at least part of the Tpr-binding region. Additionally, only minor amounts of a 120-kDa degradation product (asterisk) and some unspecific cross-reactions (u) are seen with some Nup153 antibodies. Nup98 is degraded more rapidly, with only its C-terminal domain (asterisk) resisting proteolysis slightly longer. (E) Whereas Tpr's C-terminal domain is being degraded around 8 h post-infection, its entire rod domain (double-asterisk) withstands proteolysis. The membrane marked 1622–1640 was first incubated with rb-anti-Tpr-4 (2063–2084), then stripped and re-incubated with gp-anti-Tpr-3 (1622–1640).