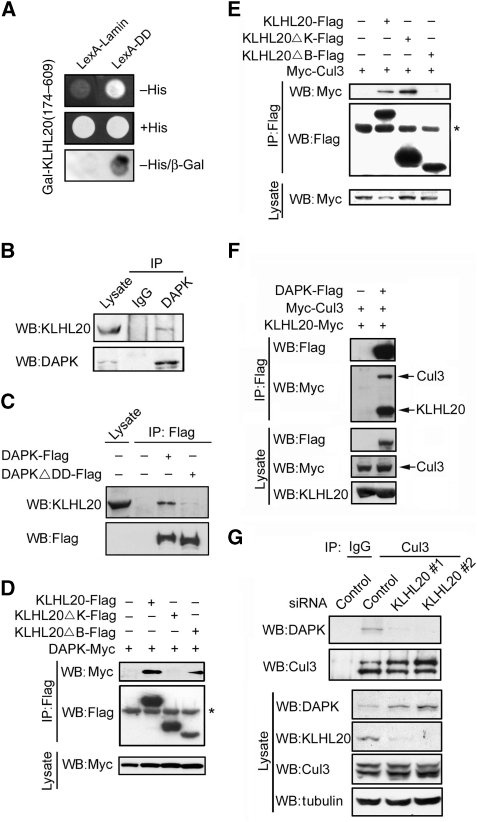
Figure 1.
Interaction of KLHL20 with Cul3 and DAPK. (A) Identification of KLHL20 as a DAPK-binding protein. Yeast strain L40 contransformed with Gal- and LexA-based constructs as indicated was assayed for His3 phenotype (−His) or β-galactosidase activity (β-Gal). (B) Endogenous KLHL20 interacts with endogenous DAPK. Lysates of HeLa cells were used for immunoprecipitation with anti-DAPK antibody or control IgG and the immunoprecipitates and cell lysate were subjected to western blot with antibodies as indicated. (C) The DD of DAPK is involved in binding KLHL20. Lysates of 293T cells transfected with DAPK-Flag or DAPKΔDD-Flag were used for immunoprecipitation and western blot analyses with antibodies as indicated. (D, E) Mapping the KLHL20 domain responsible for binding DAPK (D) or Cul3 (E). 293T cells were cotransfected with various constructs as indicated. Cells were lysed for immunoprecipitation and western blot analyses with antibodies as indicated. The position of immunoglobulin heavy chain is marked with an asterisk. (F) Cul3 coprecipitates with DAPK. Lysates of 293T cells cotransfected with various constructs were analysed by immunoprecipitation and/or western blot with indicated antibodies. (G) KLHL20 mediates the interaction between DAPK and Cul3. HeLa cells were infected with lentivirus carrying indicated siRNA and then selected with puromycin. The resulting stable knockdown cells were analysed by immunoprecipitation followed by western blot with antibodies as indicated. KLHL20, Cul3, and DAPK expression levels were assayed by western blot (bottom panel).