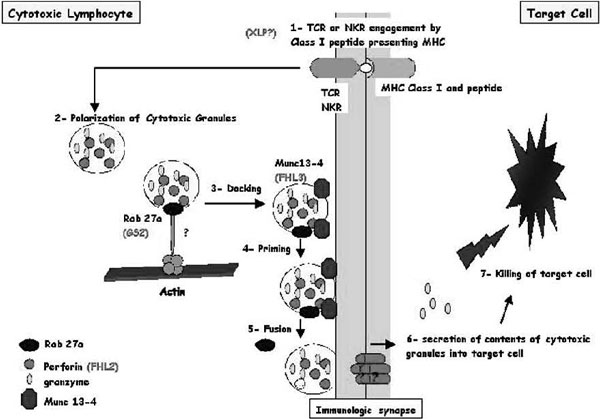
Figure 3.
Schematic representation of cytotoxic granule exocytosis and target killing following target recognition by cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells) Recognition of a peptide-major histocompatibility complex class I molecule presented by a target cell induces activation of cytotoxic lymphocytes (CTLs and NK cells). After cell conjugate formation, activated lymphocytes polarize their lytic granules toward the cell-to-cell contact, organized as an immunologic synapse. RAB27A is expected to promote the terminal transport and/or the docking step of the cytotoxic granules at the immunologic synapse. For its function, RAB27A potentially associates with unknown effectors and with MUNC13-4. MUNC13-4 functions as a priming factor, allowing cytotoxic granules to reach a fusion-competent state before membrane fusion and granule secretion occur. In 30% of patients with familial hemophagocytic lymphohistiocytosis (FHL), cytotoxic granules are defective in their functional perforin content (FHL2); in another 30% of the patients, cytotoxic granules are defective in their priming state and thus secretion (FHL3). Defective RAB27A in patients with Griscelli syndrome 2 impairs terminal transport and thus exocytosis of the lytic granule contents. X-linked lymphoproliferation and polymerization of perforin are represented with a question mark because there is no experimental proof that they act as represented in this scheme.