Fig. 7.
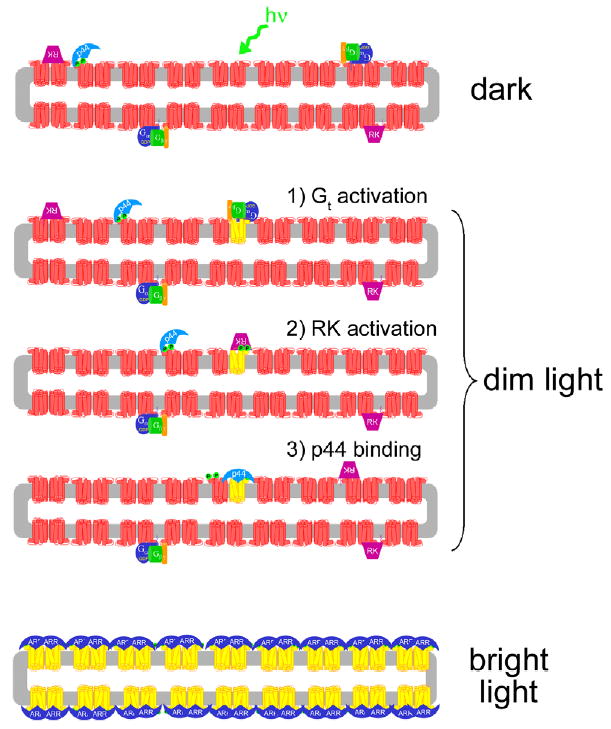
Model of light-dependent attenuation of Rho* by p44 and arrestin. In the dark-adapted rod disk (upper panel), p44 (light blue) is “tethered” to the membrane by its interaction with Rho-P (red), which comprises ~1% of the dark-state rhodopsin population (Binder et al., 1996). Transducin (Gt, blue, green, and orange subunits) and rhodopsin kinase (RK, purple) are also localized to the membrane by lipid membrane anchors (for simplicity, the interactions of RK with recoverin and Gβγ are ignored in this schematic). Upon exposure to dim light (middle panels), a single rhodopsin molecule (red) is converted to Meta II (yellow). (1) Gt binds Meta II and is activated. (2) Meta II is phosphorylated by RK. (3) p44 binds phosphorylated Meta II and blocks further Gt activation. It is not known exactly how p44 “hops” from Rho-P to Rho*-P, but rhodopsin dimerization may facilitate this process (Schröder et al., 2002). Furthermore, the rapid diffusion of rhodopsin monomeric units in the ROS membrane (Cone, 1972; Liebman & Entine, 1974) may allow p44-bound Rho-P to come into contact with Rho*-P, but this possibility is speculative. Note that different modes of p44 binding to Rho-P and Rho*-P are illustrated (see text for more details). Upon exposure to bright light (lowest panel), which results in complete rhodopsin bleaching, arrestin (dark blue) translocation from the inner segment might provide a pool of protein sufficient to bind up every photo-activated receptor (Strissel et al., 2006). In the figure, rhodopsin dimers and a binding stoichiometry of 2 arrestin to 2 Rho*-P are suggested. (For interpretation of the references to colors in this figure legend, the reader is referred to the web version of this paper.)
