Abstract
Identification of prognostic factors and risk-based post-remission therapy was proposed to improve the outcomes of acute myeloid leukemia (AML) and a mutation of FLT3 has been reported to be a risk factor, especially for pediatric patients. Recently, FLT3 expression level was implicated to have prognostic significance in adults, but little is known for childhood AML. To define the prognostic significance, transcript level of FLT3 was analyzed in 52 pediatric AML patients. The median copy number of FLT3 was 4.6×103 (40-5.9×107 copies)/1.0×106 GAPDH copy, and the relapse free survival of patients with high transcript level of FLT3 (>106 copy number) (0%) was significantly lower than that of the others (53.2%). High transcript level of FLT3 was associated with a markedly high risk of relapse. The development of new therapeutic scheme such as a frontline allogeneic stem cell transplantation or administration of FLT3 inhibitor is needed to improve outcomes.
Keywords: FLT3; Transcript Level; Leukemia, Myeloid, Acute; Pediatric Age
INTRODUCTION
Dose-intensive treatment by induction chemotherapy and post-remission therapy has brought a lot of progress in childhood acute myeloid leukemia (AML) (1), and allogeneic stem cell transplantation (SCT) with a matched related donor (MRD) is regarded as an effective post-remission therapy in pediatric patients (2-4). But the majority of patients without an appropriate related donor, received continuous chemotherapy. Because relapse remains a major cause of failure and nearly a half of patients are destined for relapse and a poor outcome after complete remission, the identification of prognostic factors and risk-based post-remission therapy have been proposed (5, 6). Although alternative donor transplants such as SCT with a matched unrelated donor (MUD) are mainly applied to restricted categories of cases (AML in second complete remission [CR], third CR or early relapse), the high risk patients are regarded to have the advantage of receiving alternative donor transplants during the first remission (7). Recently, the FLT3 internal tandem duplication (FLT3/ITD) was found to have profound prognostic effects, especially in pediatric patients (8-15) and was proposed that a patient with this mutation could be salvaged by allogeneic SCT, although there has been some controversy (6, 16-18). Transcript level of FLT3 was also proposed to have a prognostic significance in adults (19, 20), but little is known for childhood AML. In this study, transcript level of FLT3 was examined and analyzed with other prognostic factors in pediatric AML patients without very good prognostic factors such as t(15;17) and inv(16), who achieved complete remission and we proposed that high transcript level of FLT3 is associated with poor prognosis in pediatric AML.
MATERIALS AND METHODS
Patients and treatment
Transcript level of FLT3 was analyzed with the initial bone marrow sample in newly diagnosed pediatric patients (aged ≤18 yr) with AML who achieved the first complete remission. Patients with secondary AML or refroctory anemia with excess blasts in transformation (RAEB-t) were included, but patients with very good prognostic factors such as t(15;17) or inv(16) were excluded. The use of human material for scientific purposes in this study was approved by the Institutional Review Board of Seoul National University Hospital (0507-507-153).
All patients received the same treatment scheme as previously reported after informed consent (9, 21). Briefly, patients received N4-behenoyl-1-β-D-arabinofuranosyl-cytosine (BHAC), idarubicin, 6-thioguanine (6-TG), and intrathecal cytosine arabinoside (Ara-C) as an induction therapy. Primary refractory patients received a second cycle of induction therapy, excluding the 6-TG from the first regimen. Once in remission, patients with a MRD received SCT after two courses of consolidation therapy. Patents without a donor received autologous peripheral blood stem cell transplantation (APBSCT) with BCVAC (BCNU, etoposide, Ara-C, and cyclophosphamide) conditioning regimen after four courses of consolidation therapy. Recently, patient with appropriate alternative donor received unrelated SCT or cord blood transplantation.
Analysis of FLT3 mutation and transcript level
Transcript level of FLT3 was analyzed with total RNA extracted from bone marrow samples by using EASY-BLUE total RNA extraction solution (InTron Inc., Seoul, Korea) according to the manufacturer's instruction. cDNA was synthesized from each RNA (RNA measured by nano drop spect auto calculation machine; error range±0.1-0.5%) by using a RT premix kit (InTron Inc.). The transcript level of FLT3 was measured by using a real-time fluorescence detection method on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). The standard curves for FLT3 and GAPDH mRNA were generated using tenfold serial diluted FLT3 or GAPDH PCR products as template, and the copy number of FLT3 and GAPDH transcript was calculated from each standard curve. To compare the absolute expression level of each sample, the FLT3 mRNA was normalized using the expression of GAPDH gene, i.e. the number of FLT3 copies was expressed as the equivalent copy number per 106 copies of GAPDH. Each gene expression level was analyzed in triplicate, and the mean was subjected to analysis. The primer and probe sequences for RT-Q-PCR of FLT3 were sense primer, 5'-TTTCACAGGACTGGACAGAGATTT-3'antisense primer, 5'-GAGTCCGGGTGTATCTGAACTTCT-3'; and TaqMan probe, FAM-5'-CCAAATTCCAGCATGCCTGGTTCAA-3'-TAMRA. The housekeeping gene, GAPDH, served as a control for cDNA quality and normalization and the primer and probe sequences for real time quantitative PCR were sense primer, 5'-TTTCACAGGACTTGGACAGAGATTT-3'antisense primer, 5'-GAGTCCGGGTGTATCTGAACTTCT-3'; and TaqMan probe, FAM-5'-CAAGCTTCCCGTTCTCAGCC-3'-TAMRA. FLT3 mutations were analyzed as previously reported with genomic DNA isolated from bone marrow samples (9).
Statistics
Clinical and laboratory data were analyzed using standard statistical methods in SPSS version 12.0. Fisher's exact test was used to compare categorical variables among subgroups. Differences in means of continuous variables were analyzed with the Mann-Whitney U test or Kruskal-Wallis test. Relapse-free survival (RFS) was defined as the time from complete remission to relapse, and for survival analysis, Kaplan-Meier life tables were constructed and curves were compared using the log-rank test. For multivariate analysis of prognostic factors, a Cox proportional hazard regression model was used.
RESULTS
Patient characteristics
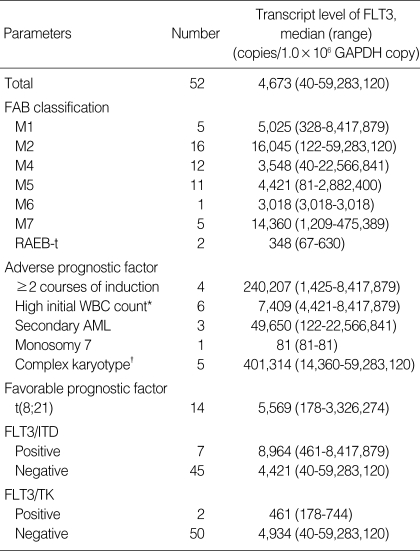
Fifty-two patients (33 males and 19 females) were analyzed in this study. The median age at diagnosis was 8 yr 5 months (4 months-17 yr 2 months). The clinical characteristics of the 52 patents are detailed in Table 1. All patients achieving CR and thirty-six patients received post-remission therapy including 16 APBSCT and 7 bone marrow transplantation (BMT) with a MRD. One patient continued to receive chemotherapy because of renal insufficiency compromising the transplant. Twelve recently diagnosed patients with appropriate alternative donors received unrelated SCT or cord blood transplantation.
Table 1.
Patient characteristics and FLT3 transcription level
*WBC count of more than 100×109/L at diagnosis; †Presence of a clone with at least five unrelated cytogenetic abnormalities.
Transcript level of FLT3
Transcript level of FLT3 was normalized with the 106 GAPDH copy number and the median level of 52 patients was 4.6×103 copies (40-5.9×107 copies). FAB subtype and risk factors of AML such as an initial high WBC count (>100×109/L), monosomy 7, more than one course for complete remission, secondary AML, MDS characteristics (RAEB-t), and FLT3/ITD were not associated with the expression level of FLT3 (Table 1).
Clinical characteristics of the patients with high transcript level of FLT3
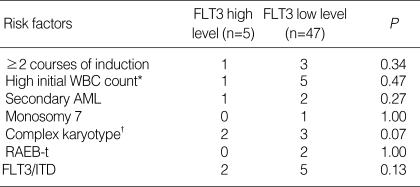
High transcript level of FLT3 (>106 copy number) was found in 5 patient (9.6%). Among them 2 patients had the FLT3/ITD mutation. High expression of FLT3 was not associated with FLT3/ITD. Four patients experienced disease recurrence before transplantation and one patient relapsed after autologous peripheral blood transplantation. The clinical data of these 5 patients are summarized in Table 2. A comparison of risk factors revealed that patients with high transcript level of FLT3 were not associated with any risk factors including FLT3/ITD (Table 3).
Table 2.
Characteristics of patients with high FLT3 level (>106 copy number)
*Relapse before post-remission transplantation.
APBSCT, autologous peripheral blood stem cell transplantation; RFS, relapse free survival.
Table 3.
Relation between high FLT3 level and other risk factors
*WBC count of more than 100×109/L at diagnosis; †Presence of a clone with at least five unrelated cytogenetic abnormalities.
Clinical outcome and prognostic significance of transcript level of FLT3
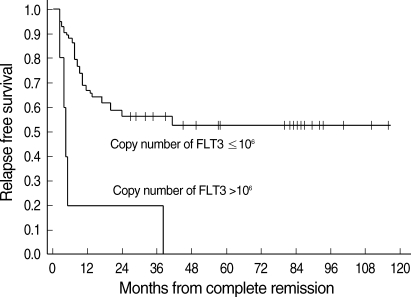
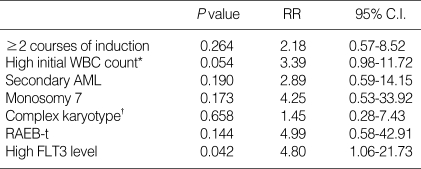
The overall survival (OS) (±standard error [SE]) and RFS (±SE) of the 52 patients were 51.0±7.9% and 46.9±7.7%, respectively. High transcript level of FLT3 (>106 copy number) was found to be a significant prognostic factor for RFS. The RFS of patients with high expression level of FLT3 (0%) was poorer than that of the other patients (53.2±8.0%) (Fig. 1), with statistical significance (P<0.001), and it was also a significant prognostic factor for RFS by multivariate analysis (P=0.042, RR=4.80 [1.06-21.73]) (Table 4).
Fig. 1.
The relapse free survival (RFS) of patients with high transcript level of FLT3 (0%) was lower than the RFS of the other patients (53.2±8.0%) (P<0.001).
Table 4.
Multivariate analysis for the prognostic factors of RFS
*WBC count of more than 100×109/L at diagnosis; †Presence of a clone with at least five unrelated cytogenetic abnormalities.
DISCUSSION
Currently, the remission rate of pediatric non-promyelocytic AML is about 70-90%, but the survival rate has been reported to be only 45-66% despite intensive post-remission therapy (22). After the great success of treatment in pediatric acute lymphoblastic leukemia (ALL) with risk based therapy, this kind of efforts are also introduced to AML (5, 6). But treatment of AML is more intensive than ALL, and there is not so much room for the stratification of treatment according to the prognostic factors in AML. In the view of urgency at diagnosis, it is somewhat hard to delay treatment in AML until cytogenetic results are confirmed. Thus, many centers start an induction regimen arranged beforehand. From this point, the prognostic factors after CR have tremendous importance.
BMT with matched sibling donor, introduction of intensive induction chemotherapy and post-remission therapy have done much to progress in the treatment of pediatric AML. Recent development of SCT with the increase of unrelated donor pool, the current proposal of risk based treatment in AML is focused on finding patients who would have a benefit from the allogeneic SCT. Some high risk factors have been established in pediatric AML. An initial high WBC count (>100×109/L), monosomy 7, more than one course for complete remission, secondary AML, prior MDS, and extramedullary leukemia (non CNS) are well known classical adverse factors (23). Monosomy 7 as well as complex karyotype, monosomy 5, deletion 5q and abnormal 3q have been proposed as adverse cytogenetic risk factors (5, 6, 24).
FLT3 is a receptor tyrosine kinase involved in the survival of hematopoietic stem cells and expressed in most acute leukemia, including more than 90% cases of AML (2, 25, 26). Mutations of FLT3 have been reported to be of prognostic significance in many studies including our previous report (8-15). But the role of allogeneic SCT in FLT3/ITD positive AML was in debate (6, 16-18).
In our previous study, FLT3/ITD was a poor prognostic factor and no patient survived (9), and FLT3/ITD also a poor prognostic factor in present study but one patient with FLT3/ITD was salvaged after cord blood transplantation.
To find another biological and clinical importance of FLT3, overexpression of FLT3 has been studied and proposed to have a prognostic significance in two adult studies (19, 20). But the prognostic role of expression level of FLT3 was not known for pediatric patients.
In the present study, FLT3 transcript level was analyzed in pediatric AML patients and high expression of FLT3 was found to have prognostic significance. Our results simply imply that patients with very high expression of FLT3 or FLT3/ITD mutation have an extremely high risk of relapse, and that this may justify the use of an alternative transplant early after CR.
FLT3 is a receptor tyrosine kinase and considered as a therapeutic target in AML. Many phase I and II clinical studies show a modest effect of small molecular compounds that directly inhibit FLT3 and preliminary reports show the combination of FLT3 inhibitors with chemotherapy was well-tolerated with encouraging rates of clinical response (27, 28). As with the promising results of incorporation of imatinib into HSCT in Philadelphia positive leukemia, this kind of agent would be incorporated into protocols for AML and hopefully improve the treatment results in high-risk patients with high expression of FLT3.
In conclusion, high transcript level of FLT3 was associated with a markedly high risk of relapse. The development of new therapeutic scheme such as a frontline allogeneic stem cell transplantation or administration of FLT3 inhibitor is needed to improve outcomes.
Footnotes
This study is supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A050089) and by grant no 04-2004-039 from SNUH Research Fund.
References
- 1.Rowe JM, Tallman MS. Intensifying induction therapy in acute myeloid leukemia: has a new standard of care emerged? Blood. 1997;90:2121–2126. [PubMed] [Google Scholar]
- 2.Amadori S, Testi AM, Aricò M, Comelli A, Giuliano M, Madon E, Masera G, Rondelli R, Zanesco L, Mandelli F The Associazione Italiana Ematologia ed Oncologia Pediatrica Cooperative Group. Prospective comparative study of bone marrow transplantation and postremission chemotherapy for childhood acute myelogenous leukemia. J Clin Oncol. 1993;11:1046–1054. doi: 10.1200/JCO.1993.11.6.1046. [DOI] [PubMed] [Google Scholar]
- 3.Ravindranath Y, Yeager AM, Chang MN, Steuber CP, Krischer J, Graham-Pole J, Carroll A, Inoue S, Camitta B, Weinstein HJ Pediatric Oncology Group. Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. N Engl J Med. 1996;334:1428–1434. doi: 10.1056/NEJM199605303342203. [DOI] [PubMed] [Google Scholar]
- 4.Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, Barnard DR, Dusenbery K, DeSwarte J, Arthur DC, Lange BJ, Kobrinsky NL Children's Cancer Group. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 5.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Oncologist. 2007;12:341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 6.Meshinchi S, Smith FO, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Curr Oncol Rep. 2003;5:489–497. doi: 10.1007/s11912-003-0010-1. [DOI] [PubMed] [Google Scholar]
- 7.Löwenberg B, Griffin JD, Tallman MS. Acute myeloid leukemia and acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2003:82–101. [PubMed] [Google Scholar]
- 8.Iwai T, Yokota S, Nakao M, Okamoto T, Taniwaki M, Onodera N, Watanabe A, Kikuta A, Tanaka A, Asami K, Sekine I, Mugishima H, Nishimura Y, Koizumi S, Horikoshi Y, Mimaya J, Ohta S, Nishikawa K, Iwai A, Shimokawa T, Nakayama M, Kawakami K, Gushiken T, Hyakuna N, Fujimoto T Children's Cancer and Leukemia Study Group, Japan. Internal tandem duplication of the FLT3 gene and clinical evaluation in childhood acute myeloid leukemia. Leukemia. 1999;13:38–43. doi: 10.1038/sj.leu.2401241. [DOI] [PubMed] [Google Scholar]
- 9.Kang HJ, Hong SH, Kim IH, Park BK, Han KS, Cho HI, Shin HY, Ahn HS. Prognostic significance of FLT3 mutations in pediatric non-promyelocytic acute myeloid leukemia. Leuk Res. 2005;29:617–623. doi: 10.1016/j.leukres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Kondo M, Horibe K, Takahashi Y, Matsumoto K, Fukuda M, Inaba J, Kato K, Kojima S, Matsuyama T. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529. doi: 10.1002/(sici)1096-911x(199912)33:6<525::aid-mpo1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Liang DC, Shih LY, Hung IJ, Yang CP, Chen SH, Jaing TH, Liu HC, Chang WH. Clinical relevance of internal tandem duplication of the FLT3 gene in childhood acute myeloid leukemia. Cancer. 2002;94:3292–3298. doi: 10.1002/cncr.10598. [DOI] [PubMed] [Google Scholar]
- 12.Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, Bernstein ID, Radich JP. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, Radich JP. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Taki T, Yang HW, Hanada R, Hongo T, Ohnishi H, Kobayashi M, Bessho F, Yanagisawa M, Hayashi Y. Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol. 1999;105:155–162. [PubMed] [Google Scholar]
- 15.Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, Podleschny M, Hählen K, Pieters R, Zimmermann M, Reinhardt D, Harbott J, Creutzig U, Kaspers GJ, Griesinger F. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 16.Bornhäuser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C AML SHG 96 study group. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. [DOI] [PubMed] [Google Scholar]
- 17.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, Linch DC. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106:3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 18.Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, Alonzo TA. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108:400–401. doi: 10.1182/blood-2005-12-4938. [DOI] [PubMed] [Google Scholar]
- 19.Kuchenbauer F, Kern W, Schoch C, Kohlmann A, Hiddemann W, Haferlach T, Schnittger S. Detailed analysis of FLT3 expression levels in acute myeloid leukemia. Haematologica. 2005;90:1617–1625. [PubMed] [Google Scholar]
- 20.Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, Miyawaki S, Kuriyama K, Shimazaki C, Akiyama H, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Ueda R, Ohno R, Emi N, Naoe T. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 21.Kang HJ, Shin HY, Choi HS, Han KS, Ahn HS. Autologous peripheral blood stem cell transplantation with BCVAC conditioning in childhood acute myeloid leukemia. Bone Marrow Transplant. 2004;33:471–476. doi: 10.1038/sj.bmt.1704389. [DOI] [PubMed] [Google Scholar]
- 22.Shenoy S, Smith FO. Hematopoietic stem cell transplantation for childhood malignancies of myeloid origin. Bone Marrow Transplant. 2008;41:141–148. doi: 10.1038/sj.bmt.1705961. [DOI] [PubMed] [Google Scholar]
- 23.Ebb DH, Weinstein HJ. Diagnosis and treatment of childhood acute myelogenous leukemia. Pediatr Clin North Am. 1997;44:847–862. doi: 10.1016/s0031-3955(05)70533-2. [DOI] [PubMed] [Google Scholar]
- 24.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A The Medical Research Council Adult and Children's Leukaemia Working Parties. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 25.Birg F, Courcoul M, Rosnet O, Bardin F, Pébusque MJ, Marchetto S, Tabilio A, Mannoni P, Birnbaum D. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80:2584–2593. [PubMed] [Google Scholar]
- 26.Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10:588–599. [PubMed] [Google Scholar]
- 27.Doepfner KT, Boller D, Arcaro A. Targeting receptor tyrosine kinase signaling in acute myeloid leukemia. Crit Rev Oncol Hematol. 2007;63:215–230. doi: 10.1016/j.critrevonc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Knapper S. FLT3 inhibition in acute myeloid leukaemia. Br J Haematol. 2007;138:687–699. doi: 10.1111/j.1365-2141.2007.06700.x. [DOI] [PubMed] [Google Scholar]