Figure 1.
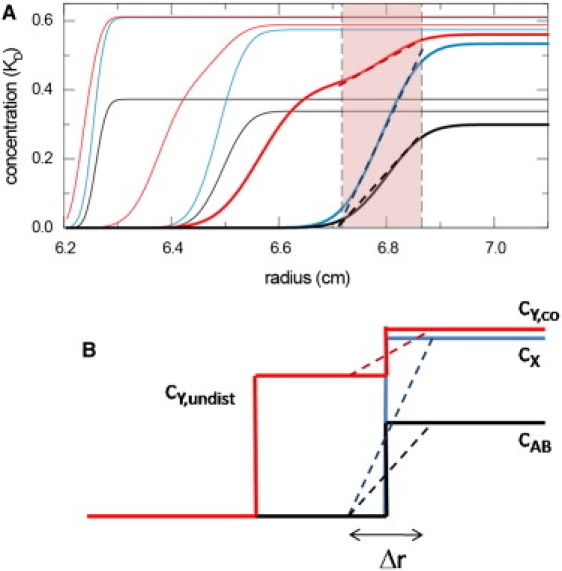
Schematic representation of the concentration gradients in the reaction boundary. (A) Concentration profiles of free A (100 kDa, 7 S, red), free B (200 kDa, 10 S, blue), and complex (13 S, black) species during the sedimentation of the interacting system A + B ↔ AB in the limit of instantaneous reaction, for the conditions at equimolar loading concentrations at KD shown in (9). For clarity, only the concentration profiles from time-points 300 s and 1500 s (thin lines) and 3000 s (bold lines) are superimposed. The vertical dashed lines and the range highlighted in red indicate the radial range that covers 10–90% of the reaction boundary at 3000 s. The dotted diagonal lines are linear approximations of the gradients in the reaction boundary. (B) Schematics of the boundary structure described in EPT, with the division of the secondary component into the undisturbed boundary with concentration cY,undist and the cosedimenting free fraction cY,co, as well as the concentration of the free species of the dominant component cX and the complex cAB in the reaction boundary. All quantities cY,undist, cY,co, cX, and cAB, as well as the question of which component plays the role of dominant and secondary component X and Y, are analytically predicted in EPT as a function of loading concentration, equilibrium constant, and all species s values. We may assign a finite boundary width Δr to the reaction boundary, and approximate it as a constant gradient.
