Figure 2.
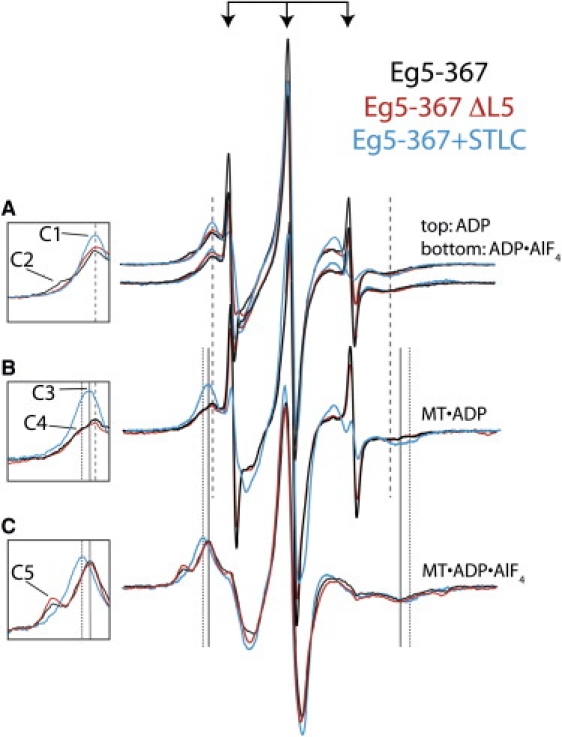
2′,3′-SLADP bound to Eg5-367 reveals mixed nucleotide pocket conformations in solution and on MTs. The innermost three sharp peaks of all spectra are from 2′,3′-SLADP that is free in solution (denoted by arrows). Insets show low-field spectral components, which are identified here by number ([C#]), as described below. (A) Top: Spectra of 2′,3′-SLADP bound to wild-type (black), ΔL5 (red), and STLC-treated Eg5-367 (blue) reveal a common inner component ([C1]) at the same magnetic field location. Bottom: Similar spectra of Eg5-367•2′,3′-SLADP•AlF4. Wild-type Eg5-367 spectra show a more immobilized component ([C2]) that is not present in STLC-treated and ΔL5 spectra. (B) When bound to MTs, 2′,3′-SLADP bound to Eg5-367 and Eg5-367 ΔL5 reveal the same inner component as in solution ([C1], coarse dashed line) as well as a broader component ([C4], fine dashed line). STLC-treated Eg5-367 displays an outward shift of a single spectral component (component [C3]). (C) 2′,3′-SLADP•AlF4 bound to Eg5-367 and Eg5-367 ΔL5 on MTs reveal identical two-component spectra. The inner component has the same low- to high-field splitting as STLC-treated MT•Eg5-367•2′,3′-SLADP ([C3], solid line). The outer component ([C5]) is from highly immobilized probes. The spectrum of STLC-treated 2′,3′-SLADP•AlF4 has a single component ([C4]) that is more immobilized than 2′,3′-SLADP.
