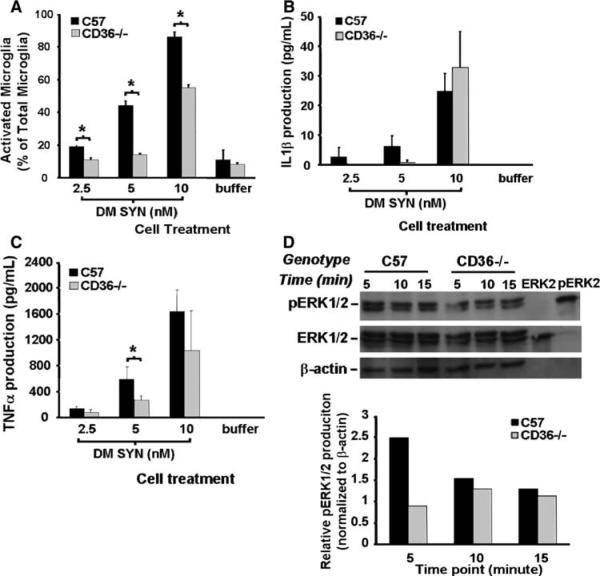
Fig. 6.
Attenuation of α-synuclein-mediated microglial activation in CD36−/− derived cultures. Primary microglia-enriched cultures were prepared from the cerebral cortices of neonatal control and CD36-null mice (C57BL/6 and CD36−/−). Cells were incubated with double-mutant α-synuclein (DM SYN; 2.5, 5, or 10 nM) or buffer alone for 24 h followed by Iba1 immunocytochemistry and enumeration of labeled cells. We observed a reduction in activated microglia from CD36−/− cultures (a;* P<0.05). Likewise, the release of IL1β and TNFα protein from microglia-conditioned media was either delayed or reduced in the CD36−/− cultures (b and c; * P<0.05). To evaluate the effect of CD36 status on phospho-ERK1/2 (pERK1/2) production, primary C57B1/6 and CD36−/− microglia-enriched cells were incubated with 10 nM DM SYN for 5, 10, or 15 min and cell lysates prepared. Cell lysates (30 μg) as well as 10 μl of purified non-phospho-ERK2 and phospho-ERK2 were analyzed by western blot analysis using anti-phospho-ERK1/2, ERK1/2, and β-actin antibodies (d) followed by densitometric analysis. This analysis demonstrated that pERK1/2 production in CD36−/− microglia at the earliest time point was 2-fold less than in similarly treated C57 microglia (d). Taken together, these data demonstrate a reduced effect of DM SYN on CD36 knock-out microglia compared to C57BL/6 microglial suggesting this scavenger receptor is in part responsible for DM SYN-dependent microglial activation