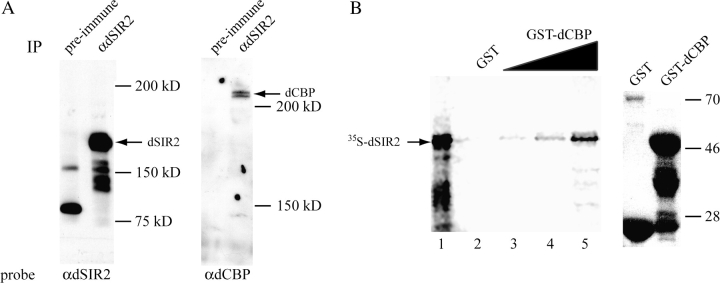
Figure 1.
dCBP and dSIR2 are in complex in vivo and directly interact in vitro. (A) Antibody to dSIR2 immunoprecipitates dSIR2 (left panel) and dCBP (right panel) from Kc cell extracts. The preimmune sera were unable to immunoprecipitate either protein (negative control). (B) The left panel shows that the dSIR2 C terminus labeled in vitro with [35S]-methionine interacts with a GST–dCBP CREB-binding domain fusion protein in a dose-dependent manner (lane 1 is the 35S dSIR2 input, lanes 3, 4, and 5 are 1, 2, and 4 μl of the dCBP–GST purified fusion protein preparation added to the 35S dSIR2). The dSIR2 protein domain does not interact with GST alone (lane 2 is 4 μl of GST protein). The right panel is a western analysis of 4 μl of the GST and GST–dCBP protein preparations used in the pull-down assays. The GST–dCBP fusion protein has a predicted molecular weight of approximately 54 kD. The lower bands are breakdown products.