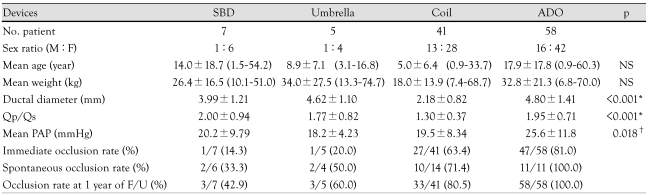
Table 1.
Demographic and catheterization data for each device group
*Significant differences between coil and other device groups, †Significant difference between coil and ADO groups. Qp/Qs: determination of the pulmonary to systemic blood flow ratio, PAP: pulmonary arterial pressure, NS: no significant difference between groups, SBD: Sideris buttoned device, ADO: Amplatzer duct occluder, F/U: follow up