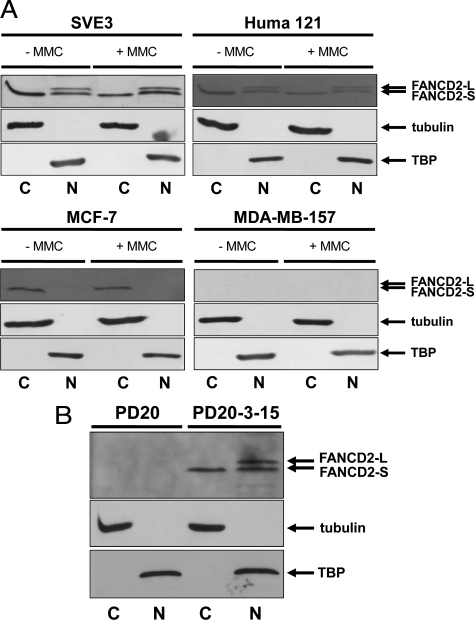
Figure 2.
Immunoblotting for FANCD2 in nuclear and cytoplasmic fractions of cultured cell lines. A: Lysates of breast cell lines SVE3, Huma 121, MCF-7, and MDA-MB-157 were fractionated into nuclear and cytoplasmic extracts and Western blotted for FANCD2 (using H300 anti-rabbit antibody). MMC treatment (+MMC) was at 50 nmol/L for 18 hours before the preparation of lysates. Nuclear (N) extracts show the presence of TATA binding protein (TBP) while cytoplasmic (C) extracts show the presence of tubulin, with no detectable cross-contamination. Immortalized normal (SVE3) and immortalized benign (Huma 121) cell lines both express immunodetectable FANCD2-S (non-monoubiquitylated; 155 kDa) and FANCD2-L (monoubiquitylated; 162 kDa) in the nucleus (irrespective of MMC-treatment), while only FANCD2-S is detectable in the cytoplasm. In the low invasive malignant cell line MCF-7, only the non-ubiquitylated FANCD2 (155 kDa) is immunodetectable and this is confined to the cytoplasm of these cells. The high invasive malignant cell line MDA-MB-157 exhibits no immunodetectable FANCD2 in either fraction. The arrows indicate the position of the marker proteins for FANCD2-L, FANCD2-S, tubulin, and TBP. B: PD20 cells (FA-D2 complementation group) and PD20-3-15 cells (PD20 functionally complemented with human chromosome 3p) were treated with 50 nmol/L MMC for 18 hours before the preparation of lysates. PD20 exhibits no immunodetectable FANCD2 protein. In PD20-3-15, both FANCD2-S (non-monoubiquitylated; 155 kDa) and FANCD2-L (monoubiquitylated; 162 kDa) are present in the nucleus, while only FANCD2-S is detectable in the cytoplasm.