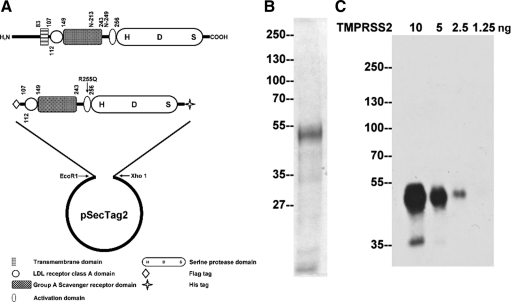
Figure 1.
TMPRSS2 recombinant protein and mAbs. A: Expression of TMPRSS2 in mammalian cells. A schematic representation of the structure of TMPRSS2 is presented at the top. The protease consists of 492 amino acids, has a calculated mass of 53,859 Da, and contains a transmembrane domain, a scavenger receptor cysteine-rich (SRCR) domain, a LDLRA domain, and a serine protease domain. The two putative N-glycosylation sites are at N-213 and N-249. The serine protease domain is at the carboxyl terminus, and the active site triad was labeled as H-D-S. A truncated and mutated TMPRSS2 expression construct was generated as described in the Materials and Methods section resulting in the construct shown. B: Purification of TMPRSS2. The recombinant TMPRSS2 protein was produced and purified from HEK 293T cells and resolved by SDS-PAGE and stained by colloidal Coomassie blue to show the protein patterns and to asses the purity of the TMPRSS2 preparation. C: Characterization of a TMPRSS2 monoclonal antibody. To evaluate the sensitivity of the TMPRSS2 monoclonal antibody, AL20, different amounts of purified TMPRSS2 protein as indicated were resolved by SDS-PAGE under reducing and boiled conditions. Immunoblot analysis was conducted with the AL20 monoclonal antibody at 2 μg/ml.