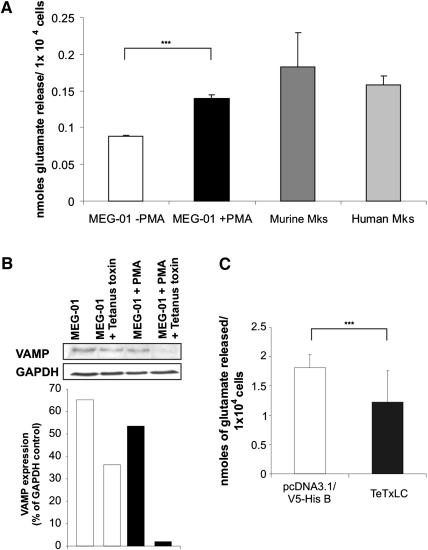
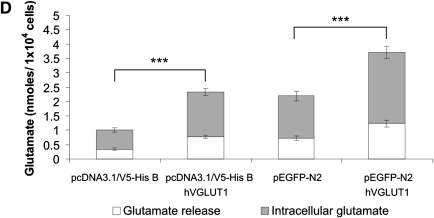
Figure 5.
Glutamate release in megakaryocytes. (A) After 72 hours of cultivation, an enzyme-linked fluorimetric assay was used to detect glutamate release from MEG-01 cells in the undifferentiated (−phorbol myristate acetate [PMA]) as well as differentiated (+PMA) state and from primary murine and human megakaryocytes (MKs) (n = 3, mean + standard deviation; ∗∗∗p < 0.001). (B) Western blot analysis (evaluated by densitometry normalized to the housekeeping protein glyceraldehyde phosphate dehydrogenase [GAPDH]) demonstrated that treatment with 100 nM tetanus toxin decreased vesicle-associated membrane protein (VAMP) protein levels in MEG-01 cells in the undifferentiated (−PMA; 45% decrease compared to undifferentiated control) as well as differentiated (+PMA; 94% decrease compared to differentiated control) state, which inhibits formation of the SNARE complex. (C) Using the enzyme-linked fluorimetric assay with MEG-01 cells overexpressing the tetanus toxin light chain (TeTxLC) demonstrated a 30% reduced glutamate release as a result of the inhibition of the SNARE complex due to reduction of VAMP compared to empty vector control (pcDNA3.1/V5-His B; n = 3, mean + standard deviation; ∗∗∗p < 0.001). (D) Two different transfection vectors (pcDNA3.1/V5-HIS B and pEGFP-N2) were used to introduce VGLUT1 overexpression into MEG-01 cells. Glutamate released from VGLUT-overexpressing cells, as well as intracellular glutamate was detected by an enzyme-linked fluorimetric assay. Compared to empty vector controls (pcDNA3.1/V5-His B and pEGFP-N2, respectively), glutamate release was 2.26- and 1.69-fold increased and intracellular glutamate was 2.29- and 1.69-fold elevated in pcDNA3.1/V-His B hVGLUT1- and pEGFP-N2 VGLUT1-transfected cells, respectively (n = 3, mean + SD; ∗∗∗p < 0.001).