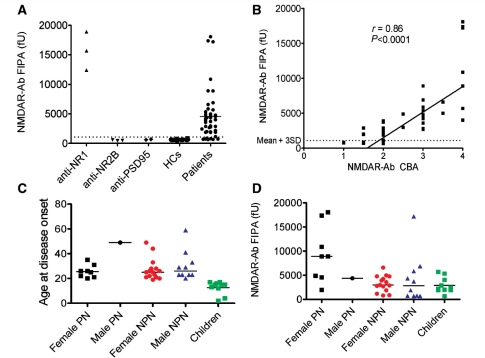
Figure 2.
Quantitative analysis of NMDAR antibodies and subgroups of NMDAR-antibody-positive patients. (A) The fluorescent immunoprecipitation assay. NR1-specific commercial antibodies (anti-NR1) precipitated large amounts of EGFP-NR1 which was not bound by NR2B-specific (anti-NR2B) or PSD95 (anti-PSD95) antibodies (data from three experiments). Eighty-four percent of 44 NMDAR-antibody-positive patients (as determined by the cell-based assay) precipitated EGFP-NR1 at levels greater than the mean plus three standard deviations (mean + 3SD) of results from 20 healthy controls (HCs). (B) There was a strong correlation between the NMDAR-antibody levels determined by the fluorescent immunoprecipitation assay and by the cell-based assay (r = 0.86, P < 0.0001; Spearman rank correlation). (C) The ages at disease onset of the 44 NMDAR-antibody-positive patients shown as male and female paraneoplastic (PN), non-paraneoplastic (NPN), and children (none of whom had tumours detected). (D) The results of the fluorescent immunoprecipitation assay showed the NMDAR-antibody levels were higher in paraneoplastic when compared to all non-paraneoplastic cases (P = 0.0017, Mann-Whitney U-test). fU = relative fluorescence units precipitated by 25 µl of serum.