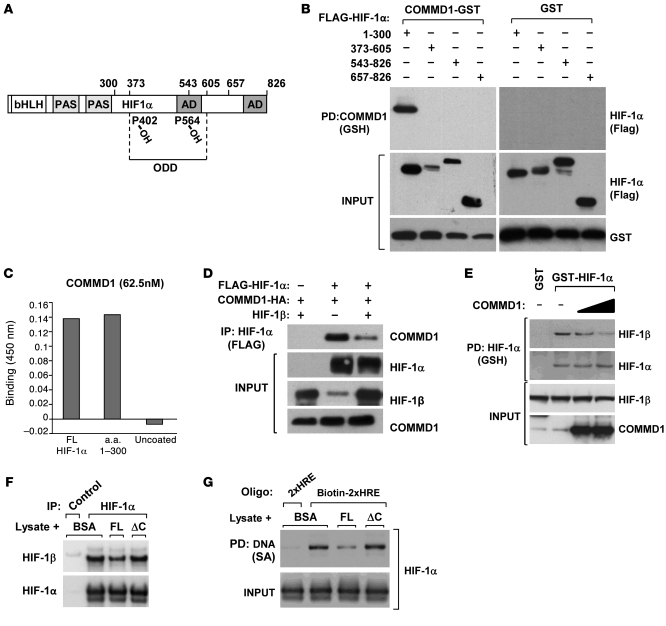
Figure 6. COMMD1 binds directly to the amino terminus of HIF-1α and inhibits HIF-1β dimerization.
(A) Schematic representation of the domains in HIF-1α. AD, activation domain. (B) HEK 293T cells expressing COMMD1-GST or GST and the indicated truncated fragments of FLAG–HIF-1α were used for GSH precipitations and Western blot analysis as shown. (C) Recombinant COMMD-1-MBP was incubated with immobilized recombinant HIF-1α full-length, HIF-1α fragment (1–300), or uncoated wells. Binding of the proteins was detected by absorbance at 450 nm, as described in the Methods section. Data represent the mean of duplicate experiments. (D) HEK 293T cells were transfected to express COMMD1-HA, FLAG–HIF-1α, or HIF-1β as indicated. HIF-1α was immunoprecipitated using an anti-FLAG antibody, and the resulting samples were subjected to Western blot analysis. (E) HEK 293T cell lysates expressing GST, GST-HIF-1α, or COMMD1 were subjected to GSH precipitations. The amount of coprecipitated endogenous HIF-1β was identified by Western blot analysis. (F and G) Lysates from HEK293 cells transfected with HA–HIF-1α were incubated with the indicated recombinant proteins. (F) Immunoprecipitation of HIF-1α (HA antibody, FLAG antibody as a control) was performed. The amount of HIF-1β recovered was assessed by immunoblotting. (G) The lysates were subsequently mixed with tandem HRE oligonucleotide probes, which were then precipitated and immunoblotted for HIF-1α. 2xHRE represents a control oligonucleotide that was not biotinylated, biotin-2xHRE represents the biotinylated version. FL, COMMD1 full-length; SA, streptavidin.