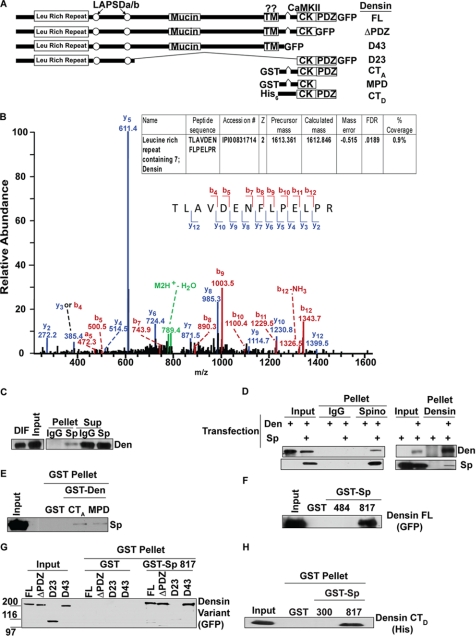
Fig. 7.
Characterization of interaction between spinophilin and densin. A, domain structure of densin and the constructs used for these experiments (LAPSDa/b, leucine-rich repeat and PDZ-specific domains a and b; Mucin, mucin homology domain; TM, transmembrane-like domain; CK, CaMKII binding region). B, MS/MS, collisionally induced dissociation, m/z spectra from the tryptic peptide matching densin is shown. The strong y-5 ion corresponds to the fragmentation after the proline residue. The inset table shows the IPI accession number, charge state of the peptide (z), precursor mass (in Da), calculated mass (in Da), mass error (in Da), and the percent coverage. C, densin is enriched in spinophilin immune complexes from striatum (Pellet) compared with the IgG control. D, GFP-densin-FL expressed in HEK293 cells co-precipitates with the spinophilin immune complex (Pellet) only when spinophilin is co-expressed; furthermore, Myc-spinophilin co-precipitates with the densin immune complex only when densin is co-expressed. E, Myc-spinophilin expressed in HEK293 cells specifically binds to GST-densin-CTA and GST-densin-MPD in a co-sedimentation assay. F, densin-FL expressed in HEK293 cells specifically associates with GST-Sp(817), but not GST-Sp(484) or GST, in a co-sedimentation assay. G, GST-Sp(817) specifically binds to densin-FL, densin-ΔPDZ, and densin-D43, but not densin-D23, in a co-sedimentation assay. H, bacterially expressed His-densin-CTD specifically binds to GST-Sp(817). DIF, detergent-insoluble fraction; Den, densin.