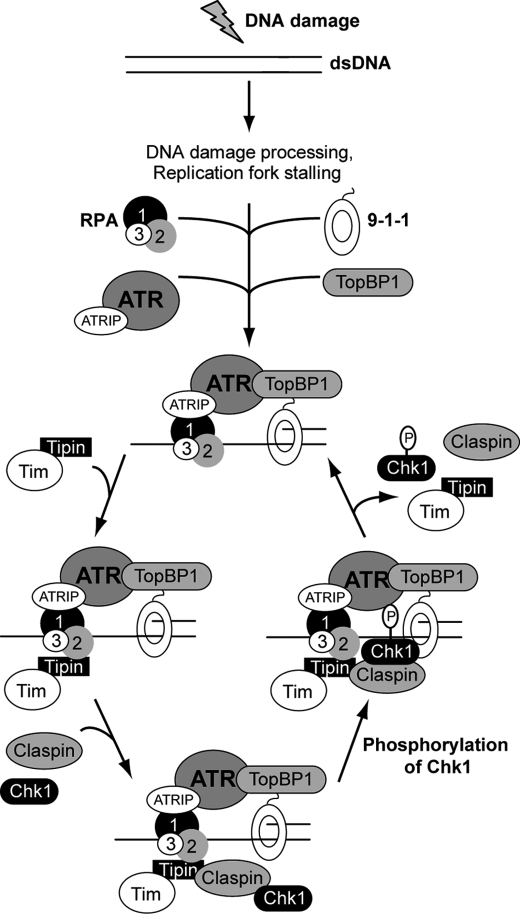
FIGURE 6.
Model for Tipin function in ATR-Chk1 signaling. In response to DNA damage or replication stress, the generation of ssDNA and presence of primer-template junctions leads to the association of RPA and 9-1-1 at sites of DNA damage. Through an interaction of the RPA1 subunit of RPA with ATRIP, the ATR kinase is recruited to these sites. Through the C-terminal domain of Rad9, TopBP1 also stably associates with damage sites, resulting in activation of ATR kinase activity. Through an interaction of the RPA2 subunit of RPA with Tipin, the Timeless-Tipin complex and then Claspin are able to associate with ATR at sites of DNA damage. The presence of Claspin allows binding of Chk1 and then phosphorylation by ATR. The data showing that Timeless-Tipin, Claspin, and Chk1 do not appear to stably associate with sites of damage in human cells indicate that these proteins may be released to allow phosphorylation of additional Chk1 molecules.