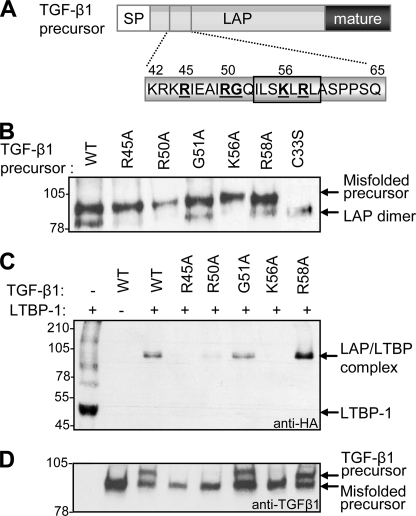
FIGURE 4.
Amino acids involved in the formation of the large latent TGF-β1 complex. Target residues in TGF-β1 LAP (boldface type, underlined) were substituted with alanine using in vitro mutagenesis (the hydrophobic residues identified to be important for LAP-mature interactions are boxed) (A). The effect of mutating these ionic residues on formation of the SLC was determined by Western blot using an anti-LAP antibody (B). The inability of the R45A, R50A, and K56A variants to fold correctly suggested that they were compromised in their ability to bind LTBPs. To verify this, cells were transfected with wild type (WT) or mutant TGF-β1 variants together with a truncated form of LTBP-1. The formation of the LLC was monitored by Western blot using an anti-hemagglutinin (anti-HA) mAb, specific for LTBP-1 (C). Expression of the TGF-β variants was verified by Western blot using a TGF-β1 mAb (D).