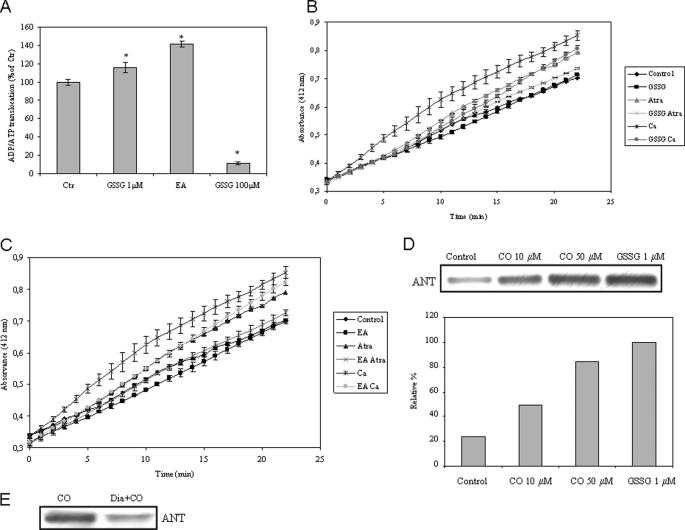
FIGURE 7.
Role of ANT glutathionylation in MMP modulation. A, ADP/ATP translocation was followed in isolated mitochondria in the presence of 1 or 100 μm GSSG or 10 μm EA. The values are expressed in relative percentage to control (100%) at 15 min of incubation at 37 °C and are mean ± S.D. (error bars), n = 3. *, p < 0.05 compared with control. B and C, isolated non-synaptic mitochondria were treated with GSSG at 1 μm (B) or with EA at 25 μm (C) for 10 min, followed by atractyloside (Atra; 300 μm) or Ca2+ (5 μm) addition in order to induce inner membrane permeabilization, which was assessed according to Ref. 29. Measurements were performed at 412 nm for 20 min at 37 °C. All values are mean ± S.D., n = 3. D, primary cultures of astrocytes were treated with 0, 10, or 50 μm CO following mitochondria isolation; additionally, 1 μm GSSG was added to mitochondria isolated from control astrocytes. Glutathionylated proteins (α-GSH) were immunoprecipitated in mitochondria isolated from astrocytes, and ANT was immunodetected by Western blot from the immunoprecipitated proteins. The area and intensity of bands were quantified by densitometry analysis (GraphPad Prism 4) and are presented as relative percentage to the positive control (100%). This experiment was repeated three times with similar results. E, isolated non-synaptic mitochondria were treated in the presence or absence of diamide at 100 μm for 15 min, followed by CO (10 μm) incubation for 15 min, and then glutathionylated proteins were immunoprecipitated, and ANT was immunodetected by Western blot from the immunoprecipitated proteins. This experiment was repeated three times with similar results.