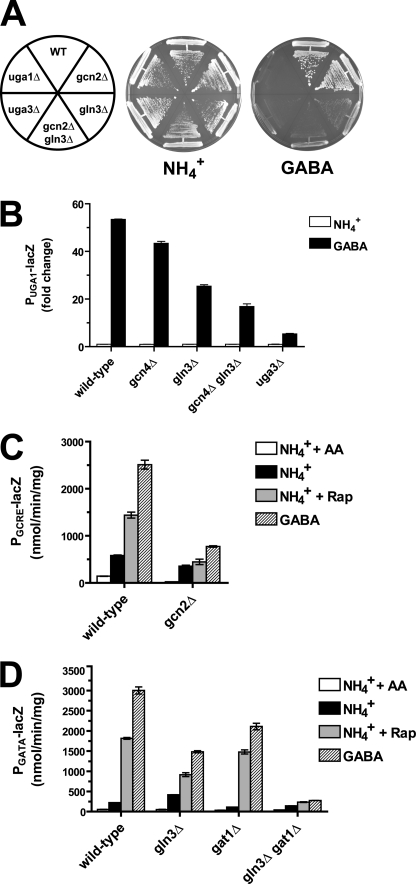
FIGURE 4.
Gcn4p and Gln3p co-regulate gene expression in response to rapamycin treatment. A, a wild-type (WT) strain and those containing the indicated gene deletions were grown on synthetic agar plates containing either ammonia (NH4+) or GABA as the nitrogen source for 3 days at 30 °C. B, yeast cells deleted for GCN4, GLN3, and UGA3, as indicated, were transformed with a plasmid encoding a lacZ reporter gene fused to the UGA1 promoter. Cells were cultured in synthetic medium containing ammonia (NH4+) as the nitrogen source and then switched to synthetic medium containing 10 mm GABA as the nitrogen source for 6 h, and β-galactosidase activity was measured. The fold change in β-galactosidase activity from cells grown in GABA medium compared with those grown in ammonia from two independent cultures ± S.E. is shown. Values are normalized to the untreated control sample in wild-type cells. C, β-galactosidase activity was measured in wild-type and gcn2Δ cells encoding PGCRE-lacZ, which were grown in synthetic medium containing ammonia as the nitrogen source in the presence (NH4+ + AA) or absence (NH4+) of all 20 amino acids. Alternatively, cells were treated with 200 nm rapamycin (NH4+ + Rap) or grown in medium containing GABA as the nitrogen source for 6 h as indicated. D, wild-type and mutant cells containing a PGATA-lacZ reporter plasmid containing the consensus GATA element were grown and assayed as described for C.